Xenotransplantation’s Second Patient: A Step Forward Through Loss
Lawrence Faucette’s sacrifice moves xenotransplantation closer to solving the global organ shortage
Jan 8, 2025
[DALL-E]
In 2023, Lawrence Faucette became the second human in history to receive a genetically modified pig heart. The 58-year-old patient, stricken with end-stage heart failure and out of options, underwent the experimental procedure at the University of Maryland Medical Center (UMMC). Forty days later, he chose to halt further treatment after his transplanted heart began to fail—a decision that would not only end his life but contribute to advancing the field of xenotransplantation.
Today, surgeon-scientists from the University of Maryland School of Medicine (UMSOM) published a detailed report in Nature Medicine analyzing the challenges, breakthroughs, and grim lessons from this extraordinary case. The report underscores the duality of hope and harsh reality in the pursuit of using genetically modified animal organs to address the severe global shortage of human donor hearts.
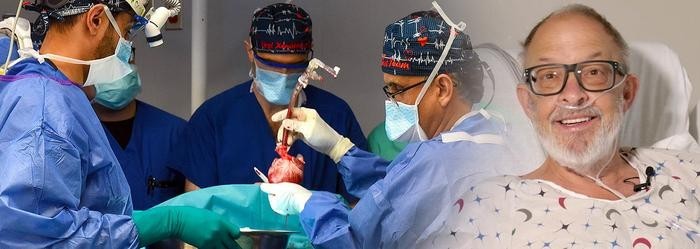
Pushing the Boundaries of Science—and Ethics
“We are humbled by what we’ve learned from Mr. Faucette’s courage and the sacrifices of his family,” said Dr. Muhammad M. Mohiuddin, one of the study’s co-leads and a leading figure in the cardiac xenotransplantation program at UMSOM. “His experience provides crucial insights into how to improve the science, particularly in overcoming the barriers of immune rejection.”
The transplant team observed signs of organ rejection just two weeks after surgery, despite rigorous immunosuppressive protocols. While the 10-gene-edited pig heart initially functioned well, a surge in anti-pig antibodies ultimately led to severe damage and graft failure. The study authors now believe more aggressive suppression of these antibodies—and possibly new strategies to modulate the immune response—will be essential in future transplants.
“This was not a failure in vain,” said Dr. Bartley P. Griffith, who performed the groundbreaking surgery. “It was a step forward in the long and arduous journey to make xenotransplantation a clinical reality.”
The Weight of Expectations
For Faucette, a traditional human heart transplant was never an option. Pre-existing conditions, including peripheral vascular disease and internal bleeding complications, excluded him from candidacy. His only lifeline was this experimental procedure. His survival, though brief, was marked by moments of medical success and a unique window into the biology of rejection.
However, the stakes in xenotransplantation go beyond individual cases. The field is emblematic of humanity’s effort to defy biological constraints. The rejection crisis Faucette faced echoes the challenges of early organ transplantation in the mid-20th century, a time when failure was the norm before eventual breakthroughs made the practice routine.
“History reminds us that progress is incremental,” said Dr. Mark T. Gladwin, Dean of UMSOM. “We must remain resolute in understanding and preventing immune-mediated rejection, even when setbacks occur.”
Scientific Lessons, Human Costs
The report’s findings expose both progress and pitfalls. Early postoperative assessments demonstrated that the pig heart achieved remarkable systolic and diastolic function, offering a tantalizing glimpse of success. However, the rapid escalation of immune rejection—despite selecting a patient with low pre-existing anti-pig antibodies—underscores the immense complexity of cross-species transplantation.
The case also highlighted the need to address other immune mechanisms. Beyond antibodies, factors like cytokine release and tissue incompatibility may have played roles in the heart’s ultimate failure.
“We are in the infancy of xenotransplantation, and there is no roadmap,” Dr. Mohiuddin acknowledged. “But these pioneering surgeries are vital. Without them, we cannot develop the tools and knowledge to save lives in the future.”
A Glimpse of the Future
The ultimate vision for xenotransplantation—a reliable and sustainable organ supply—remains tantalizingly out of reach. Yet the implications are profound. If perfected, genetically engineered organs could revolutionize healthcare, erasing waiting lists and transforming outcomes for millions of patients with organ failure.
But progress comes at a cost. Pioneering patients like Faucette and their families bear the weight of experimental failure, knowing their sacrifices may one day save others. It is a grim paradox: hope fueled by tragedy.
“We continue to learn so much from these surgeries,” said Dr. Christine Lau, Chair of Surgery at UMSOM. “This is not just about science—it’s about honoring the courage of our patients and pushing forward to solve one of medicine’s most urgent problems.”
The road ahead for xenotransplantation is long fraught with scientific and ethical challenges. But the bravery of patients like Lawrence Faucette—and the determination of the researchers and clinicians involved—ensures that the quest to rewrite the rules of organ transplantation will continue.


















