Engineered Human Therapies
When Bacteria Go Gel: The Sticky Science of Microbial Cables
Researchers have discovered that in polymer-rich environments—such as the mucus in cystic fibrosis patients—bacteria form long, tangled “cables,” a living Jell-O phenomenon that may provide new insights into infections and biofilm growth
Jan 17, 2025
[DALL-E]
Let’s start with a fun fact. You know that sticky goo called mucus, which lines your lungs and various other awkward corners of your body? It turns out that, under the right conditions, bacteria growing in that sludgy concoction can assemble themselves into long, cable-like formations. In a new paper published in Science Advances, researchers from Caltech and Princeton describe how these bacterial “cables” tangle into a bizarre, Jell-O–like web—and it’s all courtesy of spaghetti-shaped polymer molecules.
It might all sound a bit disgusting, but this observation isn’t purely academic. Take cystic fibrosis, for instance: one of its hallmark problems is especially thick, gooey mucus in the lungs, which frequently harbors stubborn and even life-threatening infections. The question is, how exactly do microbes—tiny cells only micrometers across—manage to form gargantuan tangles in that environment? And could that process reveal new ways to fight them?
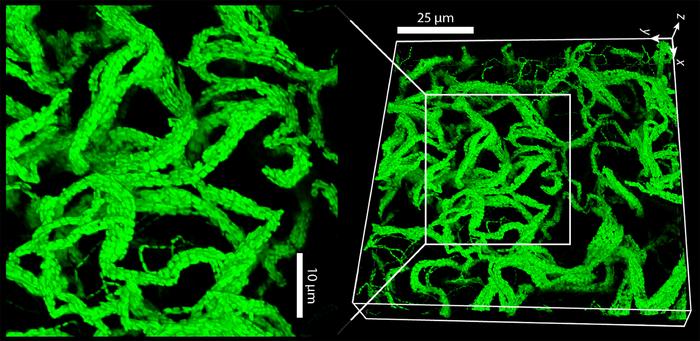
The Experimental Setup
Sujit Datta, a chemical engineering, bioengineering, and biophysics professor at Caltech, led the study. He collaborated with a graduate student from his previous research group at Princeton, Sebastian Gonzalez La Corte, to explore how bacteria behave in a mucus-like environment. The duo began by comparing bacteria grown in a standard liquid medium to those grown in samples that closely mimic cystic fibrosis mucus, courtesy of polymer-rich solutions from collaborators at MIT.
One crucial detail: their bacterial subjects—harmless lab strains of E. coli—were stripped of their ability to swim. In an ordinary watery solution, these flightless bacteria might just divide, separate, and diffuse away. But in the polymer-heavy stuff that resembles cystic fibrosis mucus, the dividing cells didn’t part ways. Instead, they stuck together end-to-end, forming what Gonzalez La Corte calls “cables”—long strands of connected bacteria.
As if that weren’t strange enough, once these cables reached a certain length, they started buckling and twisting around one another. The result was a dense, entangled mass that resembles, yes, a living gel. In fact, Datta points out that the physics of cable entanglement here is surprisingly similar to that of nonliving gels like Jell-O or even hand sanitizers.
Why All the Fuss Over Gooey Cables?
As unappetizing as this tangled bacterial mess sounds, there’s a reason scientists are paying attention. Bacteria quite often make their own polymeric matrices—biofilms, for example, that slimy residue you’ll find on river rocks, dental plaque, or worse, inside industrial equipment. These matrices are tough, sticky, and notoriously hard to get rid of. If there’s a way to disrupt how bacteria organize themselves in these polymer-dense regions, it could be a game-changer for tackling persistent infections or preventing biofilm-related damage.
Digging into the Physics
So how does it all work? In short, the dense polymeric environment exerts a gentle external pressure on the bacteria—enough to keep them mashed together. Physicists refer to this as a “depletion interaction,” where the pressure imposed by the surrounding molecules drives objects to stick together in surprising ways. Plug this into a theoretical model, and you can predict whether bacterial cables are likely to form.
Datta’s team saw the same effect with various bacterial species, in both natural and synthetic polymers. As long as there’s enough of that spaghetti-like polymer around, bacterial cells will remain glued, dividing in long chains rather than floating off as separate individuals.
The Big Biological “Why?”
That’s the million-dollar question. Is this cable formation some clever trick the bacteria have evolved, banding together to evade attacks by our immune cells (picture a hungry macrophage struggling to gobble a rope of wriggling cells)? Or is it all part of the host’s strategy—perhaps the mucus lumps the bacteria into clumps so that lung cilia can more easily sweep them away? For now, nobody really knows. But that, according to Datta, is precisely why the finding is so compelling: with the phenomenon mapped out, researchers can now design experiments to sort out whether these bacterial cables are friend or foe in the broader context of infection and immunity.
Moving Forward
The study highlights how knowledge from polymer physics—usually a domain of coffee cup lids and industrial plastics—can be applied to biological systems. It also suggests new avenues for tackling serious diseases like cystic fibrosis. Mucus, after all, isn’t just in your lungs; it’s also present in the gut and in reproductive tracts, and these bacterial tangles could be forming in many locations in the body.
Biofilms, too, are part of this conversation. They’re everywhere, from the plaque on our teeth to the slimy insides of water pipes, and their tough, polymer-laden architecture makes them infamously resistant to antibiotics. If researchers can figure out how or why bacteria adopt these cable forms, they might discover a better means of undermining that extra layer of protection.
For now, the work raises more questions than answers—exactly the kind of puzzle science is meant to tackle. As the authors note, further experiments will test whether bacteria cable up to fortify themselves or whether our bodies might be tricking them into forming bundles easier to eject. Only time (and data) will tell. Meanwhile, if you find yourself imagining your body’s mucus turning into a sort of “living Jell-O,” at least you’ll know there’s some solid science behind the picture.


















