Virus Particles Get a Bioengineered Makeover
Harnessing the power of DNA origami, researchers have reshaped viral protein assemblies, paving the way for advancements in biomedical engineering and drug delivery systems
Jul 21, 2023
DNA origami nanostructures (blue) can be used to program the shape of virus particles (grey). The native capsid with a diameter of 28 nanometres is shown in green-grey. (Mauri A. Kostiainen/Aalto University)
In a groundbreaking development, bioengineers have discovered a method to manipulate the size and shape of virus particles by integrating viral protein building blocks with DNA templates. This innovation holds promise for applications in vaccine development and internal drug transportation in the human body, marking a significant advancement in the field of bioengineering. Findings from this new study were published recently in Nature Nanotechnology.
Virus capsid proteins, the protective outer layers of a virus, can be harnessed to construct precisely formed protein assemblies. However, the shapes and geometric configurations of these assemblies are largely dependent on the specific virus strain. The potential to reprogram these assemblies irrespective of the original viral structure presents exciting possibilities for both drug delivery systems and vaccine development.
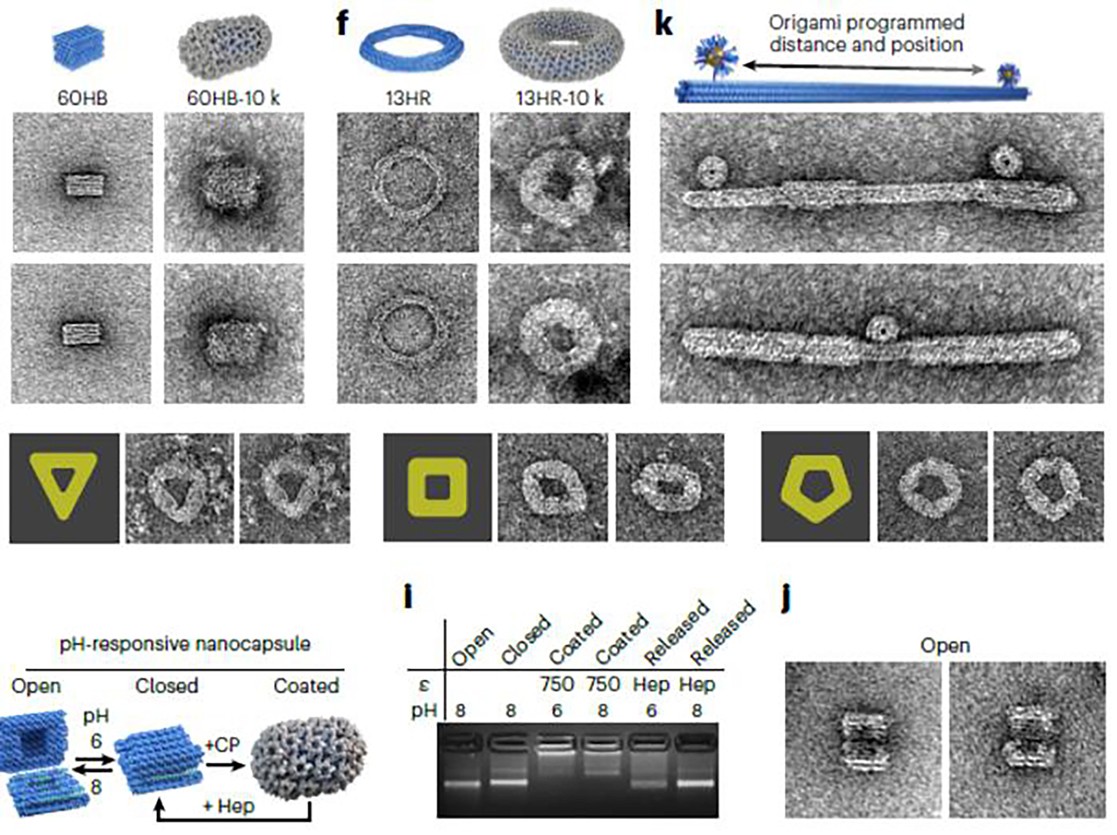
The team of scientists resolved this challenge by generating a "structured genome" template onto which the capsid proteins can form. To prevent any potential deformation of the flexible genome that could result in unintended shapes, they employed rigid DNA origami structures. Despite being only tens to hundreds of nanometres in length, these structures are entirely constructed from DNA, which is meticulously folded into the preferred template shape.
"Our strategy is centered around the electrostatic interactions between the negative charge of the DNA nanostructures and a positively charged domain of the capsid proteins, in combination with the intrinsic interactions between the individual proteins," explained Iris Seitz, the study's lead author and a doctoral researcher at Aalto University. "By varying the amount of protein we use, we can adjust the number of highly ordered protein layers encapsulating the DNA origami."
“We introduced a modular approach by demonstrating DNA-origami-directed polymorphism of single-protein subunit capsids. We achieve control over the capsid shape, size, and topology by employing user-defined DNA origami nanostructures as binding and assembly platforms, which are efficiently encapsulated within the capsid,” the authors wrote. “Furthermore, the obtained viral capsid coatings can shield the encapsulated DNA origami from degradation. Our approach is, moreover, not limited to a single type of capsomers and can also be applied to RNA–DNA origami structures to pave the way for next-generation cargo protection and targeting strategies.”
Seitz further elaborated on the innovative approach: "Using DNA origami as a blueprint, we can steer the capsid proteins to a user-defined size and shape, resulting in assemblies that are well-defined in both length and diameter. By experimenting with an array of DNA origami structures, we also discovered how the templates' geometry influenced the entire assembly."
The team used cryogenic electron microscopy imaging to visualize the highly ordered proteins upon assembly. "This allowed us to measure even minor alterations in the assembly's geometry resulting from different templates," said Professor Juha Huiskonen of the University of Helsinki, who collaborated on the study.
"We have found an uncomplicated but potent strategy to direct capsid proteins to a desired shape. Our method is adaptable and is not confined to a single capsid protein type," explained Mauri Kostiainen, an Aalto professor and the research project's leader. "We demonstrated this with capsid proteins from four different viruses. Plus, we can modify our template to be more application-relevant, for instance, by integrating RNA into the origami, which could then be translated into useful or site-specific proteins."
Despite the promise of DNA origami structures in interacting with biological systems, they have historically faced challenges in stability, particularly in the presence of DNA-degrading enzymes. In experimental trials, however, the team observed that the protein layer effectively safeguards the encapsulated DNA nanostructures from degradation.
"By merging this protective feature with the functional properties of nucleic acid origami, including the potential to deliver DNA or messenger RNA along with other cargo molecules, we believe that our approach opens up intriguing future directions for biomedical engineering," Kostiainen concluded.


















