Revolutionizing Gene Regulation: 2024 Nobel Prize in Medicine Honors the Discovery of microRNA
Victor Ambros and Gary Ruvkun's Nobel-winning research on microRNA reshaped the field of molecular biology
Oct 7, 2024
[DALL-E]
The Nobel Prize in Physiology or Medicine for 2024 has been jointly awarded to Victor Ambros and Gary Ruvkun for their groundbreaking discovery of microRNA, tiny molecules that play a critical role in the regulation of gene activity. Their findings have revolutionized our understanding of how genes are controlled at the molecular level, unveiling a new layer of complexity in gene regulation that has far-reaching implications for biology, medicine, and human health.
Unlocking the Mysteries of Gene Regulation
Every cell in the human body carries the same set of genes, yet muscle cells, nerve cells, and many others look and function very differently. This mystery, central to the science of development, boils down to how genes are regulated. Only certain genes are activated in each cell type, allowing them to perform specialized functions. This precise regulation of gene activity is essential for normal growth, development, and maintenance of bodily functions.
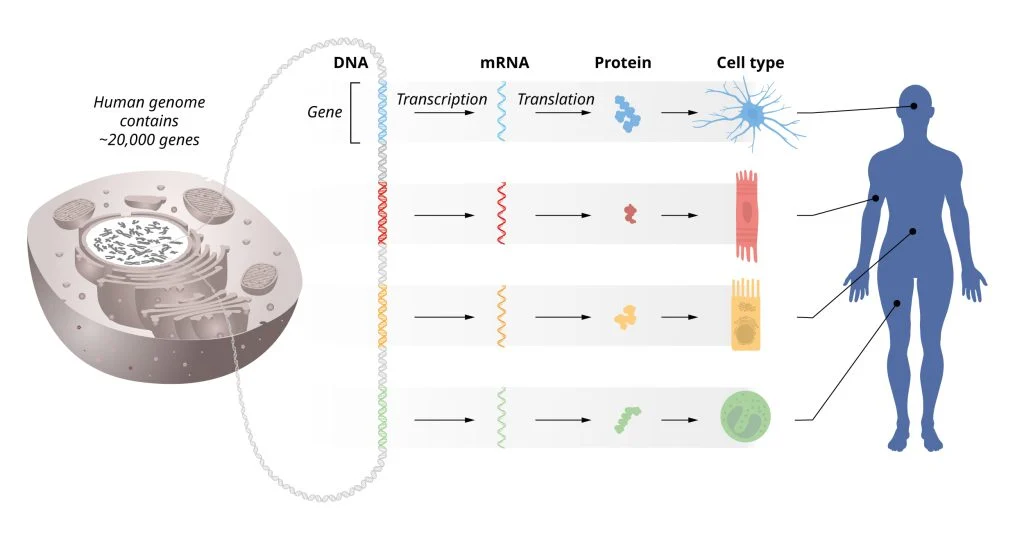
Ambros and Ruvkun's research focused on understanding how these different cell types arise, leading to the discovery of microRNAs—tiny RNA molecules that do not encode proteins but instead regulate the activity of other genes post-transcriptionally. Their work revealed that microRNAs play a crucial role in ensuring the right genes are expressed at the right time and in the right amount, adding a completely new dimension to our understanding of gene regulation.
A New Principle in Gene Control
Since the mid-20th century, scientists have known that genetic information flows from DNA to messenger RNA (mRNA) and then to proteins—the molecules that carry out essential functions in cells. But what Ambros and Ruvkun discovered was a hidden layer of control that occurs after mRNA is produced. MicroRNAs bind to complementary sequences on mRNA molecules, effectively turning off protein production by either blocking translation or leading to the degradation of the mRNA itself.
This regulatory mechanism is vital for fine-tuning gene expression across different cell types, allowing cells to adapt to changing conditions. For instance, it ensures that muscle cells produce the right proteins for contraction while nerve cells generate proteins needed for signal transmission. Without such regulation, cells would lose their functional identity, leading to developmental disorders or diseases like cancer.
A Breakthrough from Worms to Humans
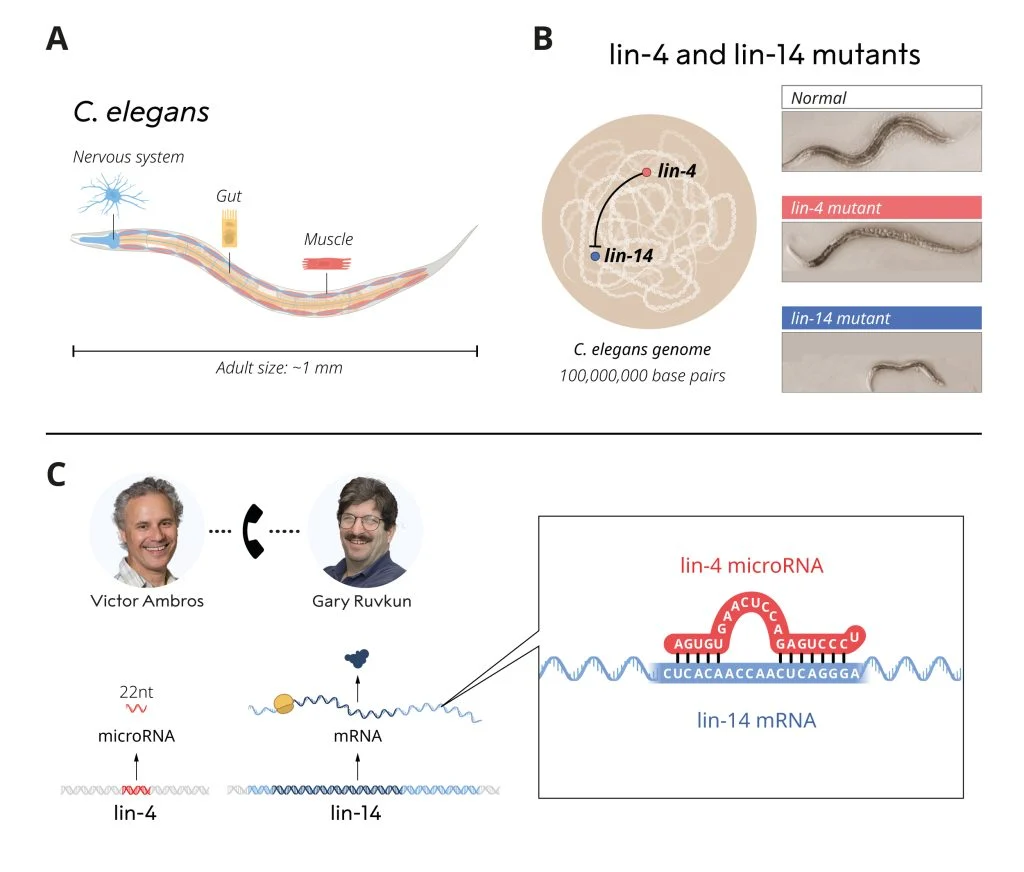
Ambros and Ruvkun's pivotal work began in the humble roundworm Caenorhabditis elegans (C. elegans), a tiny organism that has been instrumental in biological research. While studying mutant strains of C. elegans, they identified two genes, lin-4 and lin-14, which regulate developmental timing. Ambros discovered that lin-4 produces a small RNA molecule that inhibits lin-14, but in a way unlike any known mechanism at the time. Ruvkun later showed that this small RNA, a microRNA, acts by binding to complementary sequences in lin-14 mRNA, blocking protein production.
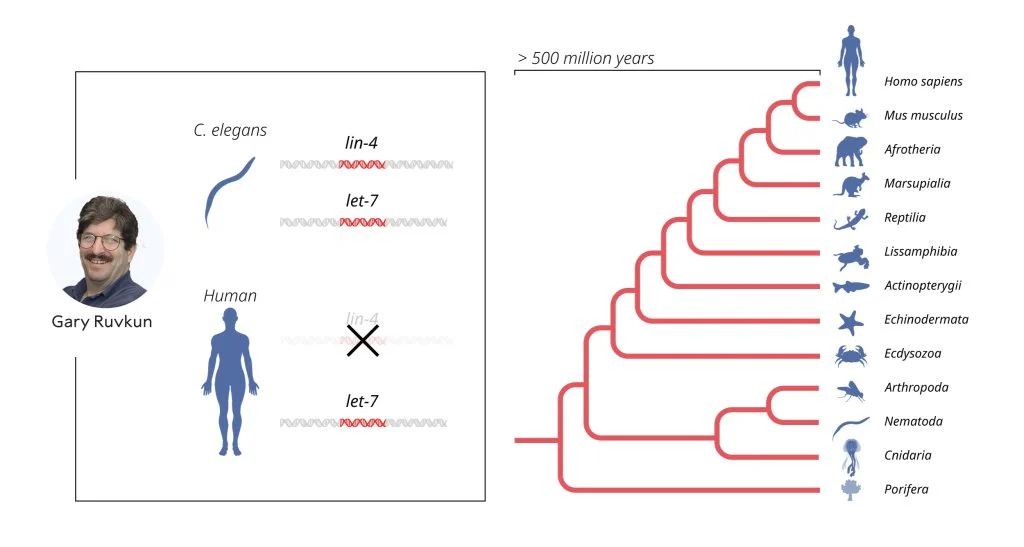
At first, the scientific community viewed this discovery as a curious finding limited to worms. But in 2000, Ruvkun's lab made another remarkable discovery: a second microRNA, let-7, which was found to be conserved across the animal kingdom, including in humans. This breakthrough opened the floodgates, and scientists quickly realized that microRNAs are present in nearly all multicellular organisms, with over a thousand now identified in humans alone.
From Discovery to Impact
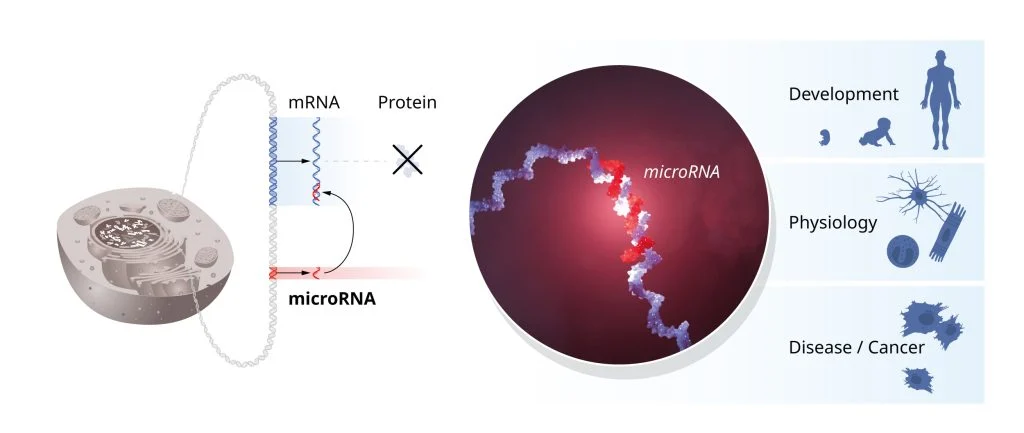
The discovery of microRNAs has reshaped the field of gene regulation, with implications that reach far beyond developmental biology. MicroRNAs are now known to be involved in many biological processes, including development, differentiation, and immune responses. Importantly, they have been linked to several diseases, such as cancer, diabetes, and autoimmune disorders, making them potential targets for therapeutic interventions.
For instance, abnormal regulation of microRNAs can lead to the uncontrolled growth of cancer cells. Mutations in microRNA-related genes have been associated with conditions like congenital hearing loss and skeletal disorders. The discovery has also spurred research into RNA-based therapies, with scientists now exploring ways to target microRNAs to treat a range of diseases.
The Legacy of Ambros and Ruvkun’s Discovery
The Nobel Prize recognizes Ambros and Ruvkun’s contributions to one of the most significant scientific advances of our time. Their discovery of microRNAs revealed a new layer of gene regulation that is essential for the development and functioning of multicellular life. From their initial work in C. elegans to the recognition that microRNA regulation is universal, their research has transformed biology and medicine.
Today, researchers continue to explore the vast regulatory networks governed by microRNAs, uncovering their roles in health and disease. Ambros and Ruvkun’s work serves as a reminder of the importance of curiosity-driven research and its potential to unlock new frontiers in science, with profound implications for human health.
Key Publications
Lee RC, Feinbaum RL, Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell. 1993;75(5):843-854. doi:10.1016/0092-8674(93)90529-y
Wightman B, Ha I, Ruvkun G. Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell. 1993;75(5):855-862. doi:10.1016/0092-8674(93)90530-4
Pasquinelli AE, Reinhart BJ, Slack F, Martindale MQ, Kurodak MI, Maller B, Hayward DC, Ball EE, Degnan B, Müller P, Spring J, Srinvasan A, Fishman M, Finnerty J, Corbo J, Levine M, Leahy P, Davidson E, Ruvkun G. Conservation of the sequence and temporal expression of let-7 heterochronic regulatory RNA. Nature. 2000;408(6808):86-89. doi:10.1038/35040556


















