Revolutionizing Drug Testing with 3D Bioprinted Vascularized Lung Cancer Models
Researchers have successfully recreated the internal environment of lung cancer patients using a novel hydrogel, paving the way for more effective treatments
Aug 16, 2023
Thomas Lauridsen (Canva)
In the bustling laboratories of Pohang University of Science and Technology (POSTECH), amid the hum of machinery and the subtle scent of chemical solutions, a breakthrough is taking shape. In its relentless pursuit of understanding, science has brought together minds from academia and industry to unlock new doors in the battle against lung cancer. The story that unfolds here is not just a chronicle of innovation; it is a testament to human ingenuity and the indomitable spirit of collaboration—the results for which can be found in the researchers' recent publication in Biofabrication.
According to data from Statistics Korea, cancer accounted for the majority of deaths in the country in 2021, constituting 26% of all recorded cases. This somber statistic is more than a mere number; it's a reflection of a continuous struggle. Similarly, cancer was the primary cause of death in 2020. Among the various types of cancer, lung cancer stands out as the most prevalent, posing significant challenges in terms of treatment due to its tendency to develop mutations that resist medication.
In the specialized world of drug screening research, such as genetic modification and targeted therapy, scientists widely employ organoids that possess the genetic characteristics of individual patients. Yet the task of replicating the intricate internal environment of a lung cancer patient's body, including the presence of tumors solely using organoids, remains formidable.
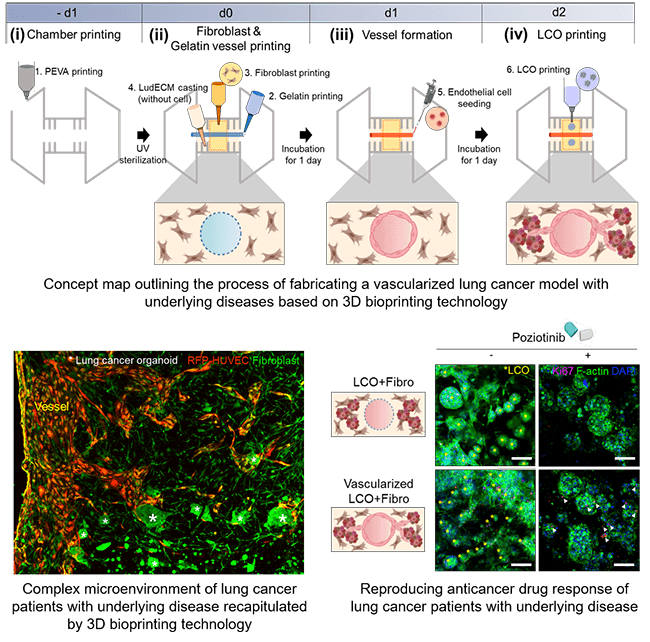
A group of researchers, including Professor Jinah Jang (Department of Mechanical Engineering and Department of Convergence IT Engineering) and PhD candidate Yoo-mi Choi from the Department of Convergence IT Engineering at POSTECH, along with Jinguen Rheey, CEO, and Haram Lee, principal researcher at Gradiant Bioconvergence, achieved a significant breakthrough. They successfully recreated the internal environment of a lung cancer patient using a hydrogel derived from the decellularized extracellular matrix (LudECM) obtained from porcine lungs.
The research team's efforts culminated in developing three distinct types of bioinks utilizing organoids derived from lung cancer patients, fibroblasts sourced from patients with underlying disease (fibrotic lung), and vascular cells. They employed LudECM as a material to replicate the intricate tumor microenvironment (TME) consisting of organoids, stromal cells, and vascular cells. Cultivating lung cancer organoids in LudECM successfully preserved the specific lung cancer subtype and genetic mutation characteristics of the patients. Notably, LudECM exhibited significantly heightened sensitivity compared to conventional Matrigel when testing drug reactions in lung cancer organoids combined with fibrosis models.
“An advanced vascularized lung cancer (LC) model is proposed, which includes patient-derived LC organoids (LCOs), lung fibroblasts, and perfusable vessels using 3D bioprinting technology,” the authors wrote. “To better recapitulate the biochemical composition of native lung tissues, a porcine lung-derived decellularized extracellular matrix (LudECM) hydrogel was produced to offer physical and biochemical cues to cells in the LC microenvironment. In particular, idiopathic pulmonary fibrosis-derived lung fibroblasts were used to implement fibrotic niches similar to actual human fibrosis. It was shown that they increased cell proliferation and the expression of drug resistance-related genes in LCOs with fibrosis. In addition, changes in resistance to sensitizing targeted anti-cancer drugs in LCOs with fibrosis were significantly greater in LudECM than in that Matrigel.”
Utilizing 3D bioprinting technology, they created a lung cancer model featuring perfusable vessels and a fibrotic lung environment. Consequently, they successfully recreated the internal environment found within the bodies of lung cancer patients with underlying diseases, now in a laboratory setting. The generated lung cancer model remarkably replicated the complex surroundings of actual tumors, thereby enabling more precise drug evaluations than previously possible.
Through meticulous experiments conducted with clinical drugs, the research team observed that a lung cancer model with fibrosis has increased drug resistance compared to a general lung cancer model. This finding underscores the potential impact of fibrotic lung on the effectiveness of anti-cancer medications. Furthermore, the team's vascularized model demonstrated instances where drug delivery could be impeded due to drug absorption into surrounding matrices or interactions between cancer cells and stromal cells. Consequently, utilizing the research team's model holds promise for providing personalized treatment options tailored specifically to lung cancer patients with underlying disease.
Professor Jinah Jang, the guiding force behind the research, explained, "Choosing appropriate anti-cancer drugs presents a challenge for lung cancer patients, given the presence of complications and associated risk factors." With a discernible sense of conviction, she continued, "I'm confident that this research will pave the way for customized treatments targeting other solid cancers that are accompanied by fibrosis."
The research team's LudECM acknowledged as a valuable biomaterial for culturing lung organoids, has garnered recognition. The technology employed for this purpose has been patented and transferred to EDmicBio, which is currently undergoing commercialization processes. In the grand tapestry of scientific advancement, this innovative chapter adds a rich texture, heralding a new era of personalized treatment in the fight against a disease that has long defied simple solutions.