Rapid Protein Production Achieved Through Biologically Engineered Reaction Crucibles
Synthetic biological compartments trap together cellular machinery to increase their rate of protein production
Feb 12, 2025
[DALL-E]
In the Crucible of Microbial Innovation
On an unassuming laboratory bench at Duke University, where the hum of incubators meets the quiet brilliance of discovery, a quiet revolution unfolds: bacteria are being reimagined as bespoke factories for proteins, even those that, in another life, might be their own undoing. In an audacious blend of synthetic biology and the artful manipulation of nature’s own blueprints, biomedical engineers have devised a method to supercharge protein production, crafting living cells that produce not only the ordinary but the extraordinary—antibiotics among them. The work, whose findings appeared online in Nature Chemistry, stands as a testament to the power of reengineering life at its most fundamental level.
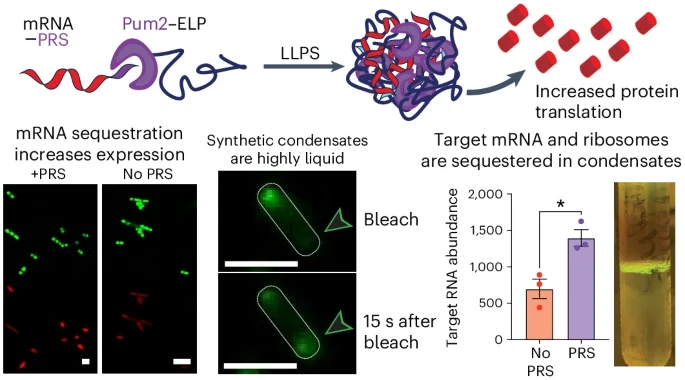
The technique directs bacteria to produce synthetic disordered proteins that bunch together to form compartments called biological condensates. These membraneless structures, assembled from carefully engineered elastin-like polypeptides—long, disordered proteins that resemble globs of noodles responsive to shifts in temperature or acidity—act as microfactories. By corralling mRNA molecules carrying the genetic blueprints alongside the cellular machinery that reads them, the condensates orchestrate a veritable symphony of protein synthesis at a pace previously unimaginable.
Microcosmic Alchemy
Nature, as it turns out, has long employed condensates as ephemeral curators of cellular function, artfully regulating gene expression in the blink of an eye. All cells, regardless of their scale, utilize these dynamic clusters to both confine and liberate biomolecular actors, thereby adjusting biochemical reactions on demand. As one PhD student put it,
“Condensates are useful for cells to temporarily control gene expression in response to new conditions or challenges because, by quickly controlling gene expression at the protein production level, rather than at the DNA level, they can make changes in which proteins are produced in minutes rather than hours or even days.”
Working under the aegis of Ashutosh Chilkoti—the Alan L. Kaganov Distinguished Professor of Biomedical Engineering at Duke—the team is among the few daring to direct cells into fabricating synthetic versions of these naturally intricate structures, tailored with a precision that nature alone could scarcely envision.
In a journey that began in earnest in 2023, the Chilkoti Laboratory pioneered the notion that bacteria might be coaxed into synthesizing these malleable protein polymers. Their early experiments demonstrated that, much like an artisan assembling parts for a delicate mechanism, cells could be induced to form condensates that modulate internal machinery. “That work showed that we, as biomedical engineers, could design new molecular parts from the ground up, convince cells to make them, and assemble these parts inside the cell to make a new machine,” Chilkoti said. “It was the beginnings of an emerging field that is now allowing us to reprogram life in new and exciting ways.”
Engineering the Reaction Crucible
Building on this innovative foundation, the latest research steers the bacterial factory with even greater finesse. The investigators have instructed the cells not only to produce the elastin-like polypeptides that form the condensates but also to imbue these compartments with the capacity to bind specific RNA sequences. By drawing these RNA strands—the very messengers that ferry protein-building instructions—into a densely populated milieu, the cell’s translation apparatus finds itself operating in a veritable crucible of concentrated reactivity. “Rather than hiding the RNA from the cell’s machinery, it seems to bring it all together at a higher concentration into a sort of reaction crucible that increases the rate of protein production,” Shapiro said. “You can make a cell express more RNA and make more of its protein, but once the RNA is made, there are very few ways of enhancing the rate at which proteins are translated from it. That’s what we did here, which is very exciting.”
This subtle manipulation of the bacterial interior has far-reaching implications. Experiments suggest that a modest tweak—designing condensates with greater viscosity—can dial down protein output, offering a precision control knob for cellular production lines. Shapiro is also exploring how the architectural nuances of the targeted mRNA influence the overall rate of translation, hinting at a future where the orchestration of gene expression may be as finely tuned as a Swiss watch.
The potential applications of this work are as diverse as they are transformative. Industries reliant on bacterial production—from pharmaceuticals to industrial chemicals and biofuels—could soon harness these engineered condensates to manufacture complex biological therapeutics more efficiently. Many life-saving treatments, including antibodies, vaccines, and immune proteins, are traditionally produced in mammalian cells due to the absence of essential biochemical machinery in bacteria. With this new strategy, however, Shapiro envisions a paradigm shift: “By using these synthetic condensates, …” a pathway may open to trap together the elusive components needed for efficient bacterial synthesis. Equally compelling is the prospect of sequestering toxic proteins—those that might otherwise compromise the bacterial host—thus overcoming a longstanding bottleneck in the production of antibiotics and other antimicrobial agents.


















