Protein Engineering of Artificial Allosteric Sites into Molecular Motors
Findings from this new study could have potential applications in industry, biology, medicine, and agriculture
Credit: salvanegra (Canva)
In a significant stride for protein research, scientists have designed artificial allosteric sites in protein complexes, paving the way for vast potential applications across industry, medicine, biology, and agriculture. The findings from the study were published recently in Nature Chemistry.
Protein complexes, the critical functional units in organisms like hemoglobin and molecular motors, accomplish their tasks via the harmonized efforts of their constituent subunits. This intricate ballet is orchestrated through a mechanism known as allostery. The process, proposed in the 1960s, remains a cornerstone in biochemistry. It involves the regulation of activity at an active site in one protein subunit by the binding of an effector molecule to an allosteric site in a different subunit.
"The creation of artificial allosteric sites into protein complexes has the potential to reveal fundamental principles for allostery and serve as tools for synthetic biology," said Professor Nobuyasu Koga from Osaka University.
The team hypothesized that protein complexes’ allosteric sites could be crafted by rekindling lost functions of pseudo-active sites, believed to have disappeared over the course of evolution. Various protein complexes possess subunits featuring these pseudo-active sites, which have been shown to possess an allosteric connection with active sites in other subunits. Thus, the team posits that unique allosteric sites could be engineered into protein complexes by retooling these pseudo-active sites.
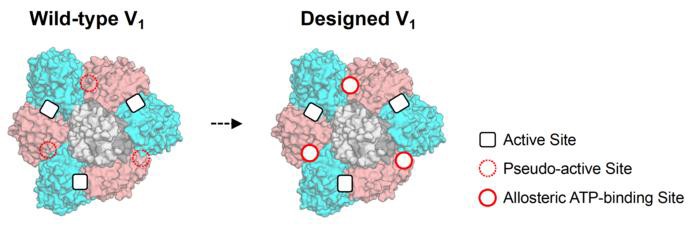
“We described an approach to create artificial allosteric sites in protein complexes. Certain protein complexes contain subunits with pseudo-active sites, which are believed to have lost functions during evolution,” the authors wrote. “Our hypothesis is that allosteric sites in such protein complexes can be created by restoring the lost functions of pseudo-active sites. We used computational design to restore the lost ATP-binding ability of the pseudo-active site in the B subunit of a rotary molecular motor, V1-ATPase. Single-molecule experiments with X-ray crystallography analyses revealed that binding of ATP to the designed allosteric site boosts this V1’s activity compared with the wild-type, and the rotation rate can be tuned by modulating ATP’s binding affinity.”
Their computational designs restored the lost ATP-binding capability of the pseudo-active site in a particular rotary molecular motor, the V1-ATPase. Successful integration of the designed site into the natural protein was validated via X-ray crystallography.
"The X-ray structure indicated the binding site is successfully designed and integrated into the natural protein to have a function. I was amazed at the utility with high performance of protein design technology," said Mikio Tanabe, an Associate Professor at the Institute of Materials Structure Science.
Further experiments revealed that the ATP binding to the designed allosteric site improved the activity of the V1-ATPase, even allowing its rotation rate to be controlled by modulating ATP's binding affinity. This enhancement of activity is a notable first in protein engineering, according to Professor Ryota Iino from the National Institutes of Natural Sciences.
"Pseudo-active sites are widespread in nature, and our approach demonstrates their potential as a means to program allosteric control over protein complexes' concerted functions," stated Assistant Professor Takahiro Kosugi, also from the National Institutes of Natural Sciences. He went on to express ambitions for the team's next steps, stating, "We hope allosteric control over concerted functions of protein complexes will open up new avenues in industrial applications of enzymes or biological, medical, and agricultural fields."


















