Nanopore-Nanobody Interaction Could Be Key to Rapid, Accurate Disease Diagnostics
Jun 17, 2023
Credit: Jonathan Bailey, NHGRI. (Flickr)
In an innovative endeavor that could transform the realm of medical diagnostics, researchers from Aarhus University and Groningen University have reached a significant milestone in developing nano-sized pores. These minuscule creations have the potential to enhance disease detection, potentially identifying conditions in their early stages. The recent publication of their work in ACS Nano details an ingenious method for identifying specific proteins in complex biological fluids, such as blood, without the need for chemical labeling.
Guided by Professor Jørgen Kjems of Aarhus University and his counterpart at Groningen University, Giovanni Maglia, the team has successfully evolved the use of nanopores, or miniature channels, in materials used as sensors. They achieved this by designing a novel type of nanopore called ClyA with attached scanner molecules, or nanobodies. Nanobodies are derived from antibodies and demonstrate remarkable precision in identifying different proteins.
These ClyA nanopores, equipped with nanobodies via a DNA adapter, have been harnessed to create a series of different nanopore sensors. These sensors can detect various proteins varying in size and function.
Of noteworthy achievement, the team constructed nanopores that could identify the SARS-CoV-2 Spike protein and a protein marker associated with breast cancer, urokinase-type plasminogen activator (uPA). They accomplished this by observing alterations in electrical currents caused by the presence of these proteins, enabling them to pinpoint individual proteins and ascertain their concentration levels. Notably, the accuracy and sensitivity of these nanopores prevailed, even when subjected to complex samples such as blood.
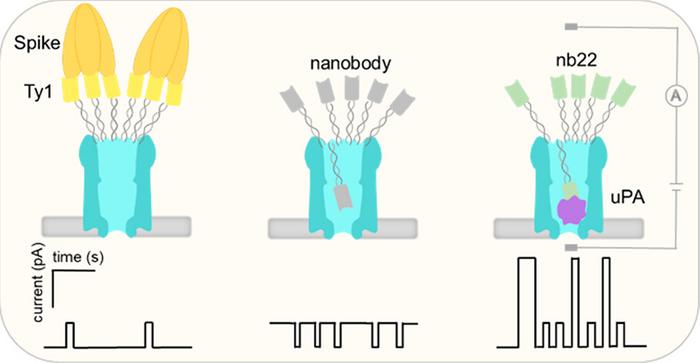
“We described a ClyA nanopore functionalized with different nanobodies through a 5–6 nm DNA linker at its periphery. Ty1, 2Rs15d, 2Rb17c, and nb22 nanobodies were employed to specifically recognize the large protein SARS-CoV-2 Spike, a medium-sized HER2 receptor, and the small protein murine urokinase-type plasminogen activator (muPA), respectively,” the authors wrote. “The pores modified with Ty1, 2Rs15d, and 2Rb17c were capable of stochastic sensing of Spike protein and HER2 receptor, respectively, following a model where unbound nanobodies, facilitated by a DNA linker, move inside the nanopore and provoke reversible blockade events, whereas engagement with the large- and medium-sized proteins outside of the pore leads to a reduced dynamic movement of the nanobodies and an increased current through the open pore.”
The researchers went on to explain that “exploiting the multivalent interaction between trimeric Spike protein and multimerized Ty1 nanobodies enabled the detection of picomolar concentrations of Spike protein. In comparison, detection of the smaller muPA proteins follows a different model where muPA, complexing with the nb22, moves into the pore, generating larger blockage signals.”
Although microscopic, the implications of this research are colossal. Existing technology allows for the incorporation of nanopores into handheld devices capable of scanning fluids for specific molecules. This opens the door to a future where diseases such as cancer and infectious conditions can be rapidly and accurately detected with a straightforward blood test. This breakthrough could enable earlier interventions, enhance treatment results, and, overall, revolutionize healthcare.
While further research and validation are necessary before the technology can be broadly implemented, this joint university collaboration propels us toward a new healthcare horizon. This breakthrough underlines the transformative potential of scientific collaboration and innovation in reshaping the healthcare landscape.