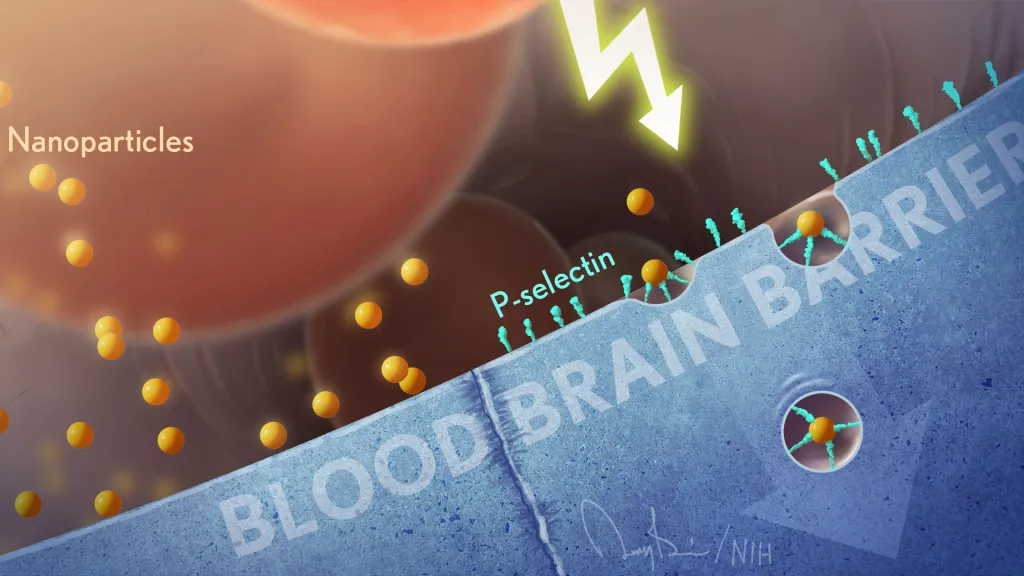
Nanoparticles with a Mission: How Peptides Are Unlocking Brain-Specific Treatments
Penn Engineers have developed lipid nanoparticles that can cross the blood-brain barrier and deliver mRNA directly to neurons, paving the way for revolutionary treatments for Alzheimer’s and Parkinson’s
Dec 17, 2024
[NIH]
Imagine a fleet of microscopic couriers slipping past the brain’s most impenetrable defenses, delivering life-saving instructions directly to the cells that need them most. Penn Engineers have achieved exactly that, modifying lipid nanoparticles (LNPs) to not only breach the blood-brain barrier (BBB)—one of medicine’s toughest obstacles—but to pinpoint specific brain cells, such as neurons. This breakthrough, detailed in Nano Letters, opens new doors for precision treatments of devastating neurological diseases like Alzheimer’s and Parkinson’s.
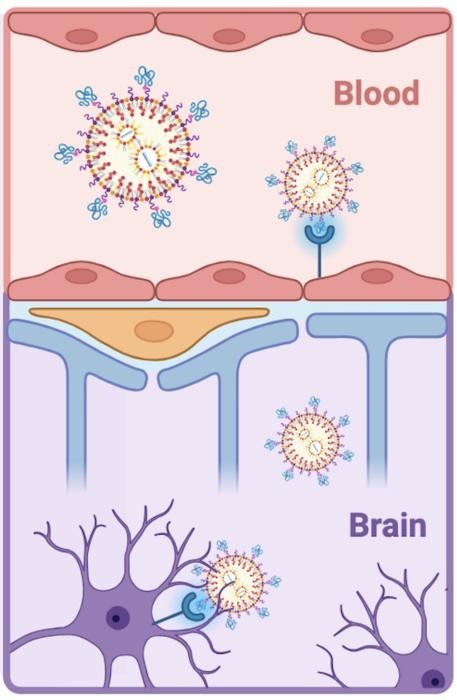
LNPs gained prominence as the delivery vehicle behind the COVID-19 mRNA vaccines. But until now, researchers have faced significant challenges in directing these nanoparticles to precise targets within the brain. Leveraging peptides—short amino acid chains—Penn researchers have solved this targeting problem, enabling mRNA delivery to endothelial cells lining brain blood vessels and neurons.
“Our first paper was a proof-of-concept lipid nanoparticle design,” explains Michael J. Mitchell, Associate Professor of Bioengineering and senior author of the study. “It was like showing we could send a package from Pennsylvania to California, but we had no idea where in California it would end up. Now, with peptides, we can address the package to specific destinations with shared features, like every house with a red mailbox.”
Cracking the Brain’s Security System
The blood-brain barrier presents one of the most formidable challenges in medicine. Designed to protect the brain from harmful substances, it blocks almost all large or foreign molecules, including pharmaceuticals and mRNA. The BBB not only prevents access but actively ejects unwanted materials. Current solutions, such as injecting treatments directly into the brain or spinal cord, are invasive and impractical.
“You can inject a treatment directly into the brain or spine, but these are highly invasive procedures,” says Emily Han, a doctoral student in the Mitchell Lab and the study’s first author.
The Penn team overcame this barrier using LNPs, which are partially composed of fatty molecules—similar to those in oils—that can bypass the BBB. By functionalizing these LNPs with peptides, the researchers created a delivery system that not only enters the brain but also targets specific cells with precision.
Why Peptides Over Antibodies?
Traditional efforts to target LNPs have relied on antibodies, large proteins that act as biological “nametags” to direct therapies to specific organs. However, antibodies add size and instability to LNPs, limiting their ability to slip through the BBB.
Peptides offer a better alternative. Composed of just a few dozen amino acids, they are smaller, cheaper to manufacture, and less likely to provoke immune responses. Their compact size allows them to be incorporated into LNPs without aggregation, maintaining the particles' stability and targeting precision.
This breakthrough began with a surprising personal experience. While researching the rabies vaccine after an encounter with a bat, Han discovered the rabies virus glycoprotein—a molecule that naturally crosses the BBB. From this, she identified RVG29, a 29-amino acid peptide segment capable of targeting brain cells.
Building and Testing Targeted LNPs
Developing and testing peptide-functionalized LNPs (pLNPs) was a meticulous process. The researchers first verified that peptides adhered properly to the LNPs, a challenge given the complex mix of nucleic acids, lipids, and peptides involved.
“Our LNPs are a complex mixture,” says Han. “We had to optimize quantification methods to pick out the peptides against all those other signals.”
To confirm the system’s targeting accuracy, the team tested pLNPs in animal models. This required a labor-intensive protocol to disassemble brain tissue and analyze cell types—a six-month effort akin to a mechanic carefully taking apart an engine.
Toward Next-Generation Neurological Therapies
The team now aims to determine how many neurons must be treated to achieve therapeutic benefits for neurological diseases.
“Returning to the same analogy,” says Mitchell, “do we need to send these to every house with a red mailbox, or just 10% of them? Would 10% of neurons be enough?”
Answering this question will allow researchers to optimize delivery strategies, bringing targeted mRNA therapies for Alzheimer’s, Parkinson’s, and other neurodegenerative diseases closer to reality.
Potential Impact
This precision-targeting technology could be a game-changer for neurological disease treatment. By delivering therapeutic mRNA specifically to affected brain cells, Penn’s approach minimizes off-target effects and opens new avenues for non-invasive, effective therapies.
The future of neurological medicine may soon rely on lipid nanoparticles, small but powerful vehicles with the ability to navigate the brain’s defenses and deliver life-changing therapies to the cells that need them most.