Light-Powered Organoids Illuminate Nervous System Development
Researchers at the Max Delbrück Center have innovatively combined spatial transcriptomics with optogenetics to manipulate gene expression in organoids using light, potentially revolutionizing precision therapies and medical research
Nov 1, 2023
AI Image Created with DALL-E 3
Scientists at the Max Delbrück Center (MDC) have unveiled a pioneering method for manipulating the development of organoids, three-dimensional cell cultures that play a key role in medical and clinical research. These miniature organs are able to replicate tissue structures and organ functions in a petri dish and hold immense promise for advancing our understanding of diseases, organ development, and the action of pharmaceuticals. Using spatial transcriptomics, researchers can observe spatial and temporal gene activity to gain a deeper insight into the inner workings of these cells.
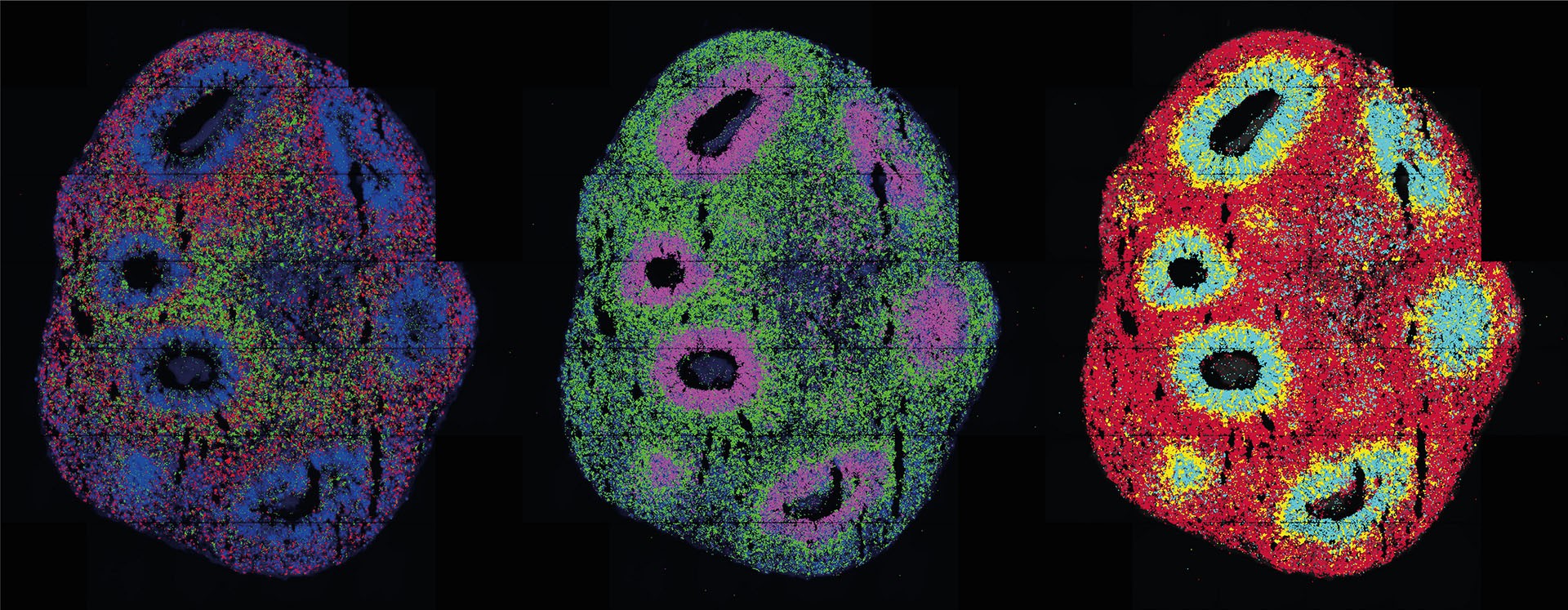
Organoids are usually derived from stem cells, versatile cells that haven’t differentiated at all or only minimally, meaning that they may become any kind of cell with the right trigger. To control cell differentiation, cells are fed growth factors and embedded in a nutrient solution. Eventually, the cells bunch together and begin functioning as if they were in a real tissue. Previously, this process was almost impossible to control. However, in a paper published in Nature Methods, researchers led by Professor Nikolaus Rajewsky, Director of the Berlin Institute for Medical Systems Biology of the Max Delbrück Center (MDC-BIMSB), have revealed a new technology to not only initiate and control the differentiation process but also monitor it in both space and time. “We combined spatial transcriptomics with optogenetics,” says lead author Dr. Ivano Legnini. “This allows us to both control gene expression in living cells and observe their behavior."
Optogenetics involves introducing light-sensitive sensors into cells, enabling the activation or inhibition of specific genes when exposed to light. Legnini applied this technique to stem cell-derived neuronal precursor cells, which were destined to form neural organoids. This work, in collaboration with the Organoid Technology Platform team, led by Dr. Agnieszka Rybak-Wolf, and the Systems Biology of Neural Tissue Differentiation Lab, led by Dr. Robert Patrick Zinzen, aimed to find out how the nervous system develops in the human embryo. This process is driven by molecules known as morphogens, which signal to neuronal progenitors where they are destined to function, for example. These are mixed and matched to produce typical patterns of gene expression during development.
The researchers succeeded in using light to activate a crucial morphogen called Sonic Hedgehog. The subsequent spatially resolved single-cell analyses revealed a remarkable result: the cells organized themselves into stereotypically patterned organoids, mimicking natural development processes. This was achieved using two distinct methods to produce light patterns – a laser microscope and a digital micromirror device, the latter of which was developed in collaboration with Dr. Andrew Woehler, who currently leads the Janelia Experimental Technology facility at the Howard Hughes Medical Institute in Ashburn, Virginia. The micromirror microscope, equipped with a chip containing hundreds of thousands of tiny mirrors, allows for complex light patterns on a sample. This technology offers high precision in replicating gene expression processes within tissues.
“Our method allows us to very accurately reproduce, in the petri dish, processes that are connected to gene expression in tissue,” says Legnini. Currently establishing his own working group at the Human Technopole in Milan, Italy, he aims to further enhance the technology's spatial and temporal resolution, expanding its applicability to a wider range of organoids.
Rajewsky also wants to refine the method: “I’m really looking forward to working with optogenetics experts to further improve the technology and to apply it to clinically relevant human organoid models.”
The Max Delbrück Center for Molecular Medicine, one of the world's premier biomedical research institutions, has been at the forefront of this transformative research. Founded by Nobel laureate Max Delbrück, the MDC is committed to unraveling the mysteries of human biology, from the most fundamental cellular processes to comprehensive system-wide mechanisms. This work promises to bring these discoveries to patients as quickly as possible to advance our ability to prevent, diagnose, and treat diseases with tailored therapies.


















