Engineered Human Therapies
Harnessing Bacteriophage as a New Weapon Against Acute GVHD
New research uncovers the potential of endolysin enzyme in combating multidrug-resistant infections in allo-HCT patients
Jul 10, 2024
[Science Photo Library/Canva]
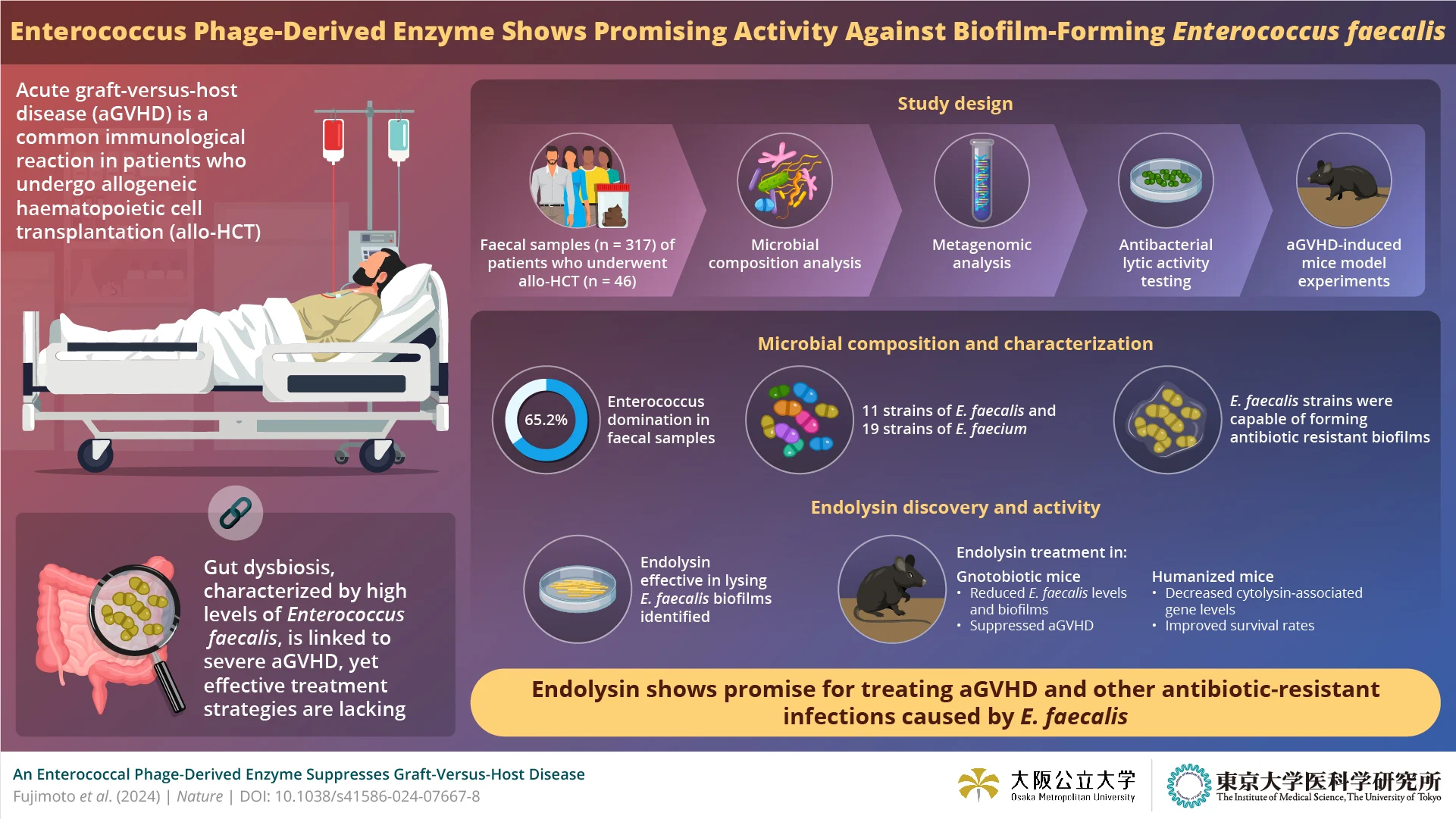
Allogenic hematopoietic cell transplantation (allo-HCT) has become a cornerstone treatment for various severe conditions, such as blood cancer, bone marrow failure, and specific genetic blood disorders. However, a common and severe complication—acute graft-versus-host disease (aGVHD)—poses significant challenges. This occurs when the donor's immune cells attack the recipient's tissues. Recent research highlights the critical role of the microbiome in developing aGVHD, with dysbiosis (microbial imbalance) playing a pivotal role. Dysbiosis can lead to the emergence of pathogenic bacteria like Enterococcus species, particularly E. faecalis and E. faecium, notorious for causing multidrug-resistant infections in allo-HCT patients. Despite its importance, there is a notable lack of therapies tailored to address dysbiosis in the context of aGVHD.
A multidisciplinary team, spearheaded by Associate Professor Kosuke Fujimoto from Osaka Metropolitan University and The University of Tokyo, in collaboration with Professor Seiya Imoto from The University of Tokyo and Satoshi Uematsu from Osaka Metropolitan University and The University of Tokyo, undertook an in-depth analysis of the intestinal bacteriome in allo-HCT patients. Their study aimed to understand the prevalence and implications of Enterococcus domination in this patient population. Their groundbreaking findings, published recently in Nature, provide new insights into the dynamics of gut microbiota in allo-HCT, inspiring a new wave of research and potential treatments in the field.
"During dysbiosis, some symbiotic, commensal bacteria acquire pathogenic traits, proliferate, and become directly involved in the onset and progression of diseases," explains Fujimoto. "Recognizing the specificity of phage therapy and its ability to spare beneficial bacteria from adverse effects, we focused our research on phage-derived lytic enzymes."
The researchers began by examining the intestinal microbiome of allo-HCT patients, identifying a dominance of Enterococcus species, especially E. faecalis, which was closely linked to acute leukemia cases. Despite sensitivity to several antibiotics, E. faecalis strains carried cytolysin-associated genes, indicating high virulence. Metagenomic analysis further revealed genetic markers for biofilm formation. Whole-genome sequencing of E. faecalis uncovered an intriguing bacteriophage-derived enzyme, endolysin, which exhibited potent antibacterial activity specifically against E. faecalis.
Rigorous in-vitro and in-vivo assays confirmed the efficacy of the endolysin. The enzyme demonstrated narrow-spectrum activity against E. faecalis, effectively lysing biofilms without affecting other intestinal bacteria. In mouse models, the endolysin's efficacy was tested in two experiments. In the first, mice with induced aGVHD treated with endolysin showed a significant reduction in E. faecalis colonization in their feces and a suppression of aGVHD development. In the second, mice with a human-like gut microbiota dominated by Enterococcus bacteria were treated with endolysin, resulting in decreased Enterococcus levels and improved survival rates.
"Bacteriophage research is gaining momentum, with advancements in phage therapy paving the way for new treatments. Our discovery of the endolysin enzyme holds promise for future applications in preventing or treating acute GVHD," says Fujimoto, expressing his optimism about their research's potential impact and real-world applications.
Thanks to this innovative research team's identification of endolysin from bacteriophages, a new class of therapeutic compounds targeting highly resistant, biofilm-forming bacteria is now on the horizon. This breakthrough opens new avenues for combating infections and complications in allo-HCT patients, offering hope for improved outcomes and enhanced quality of life. The potential impact of this research on patient outcomes is significant, providing reassurance and confidence in the future of treatment for allo-HCT patients.


















