‘Fixing’ Nitrogen: Can Engineered Microbes Replace Synthetic Fertilizers?
Reliance on synthetic fertilizers is reaching a tipping point, so scientists are turning to genetic engineering to provide a sustainable solution to feed the future
Sep 26, 2023
Oticki (Canva)
It’s no secret that synthetic nitrogen fertilizers are unsustainable, and with a growing population to feed, farmers are growing increasingly dependent on these chemical inputs. In a bid to alleviate this issue, a consortium of bacteriologists and plant scientists has outlined an innovative approach to supply the world's food crops with the nitrogen they need. The proposal, elucidated in a review in Trends in Microbiology, spotlights the potential of genetic engineering to cultivate symbiotic relationships between plants and nitrogen-fixing microbes known as "diazotrophs." This engineered harmony seeks to emulate the natural mutualistic associations seen in legumes and nitrogen-fixing bacteria, enabling crops to harness atmospheric nitrogen.
“Engineering associative diazotrophs to provide nitrogen to crops is a promising and relatively quickly realizable solution to the high cost and sustainability issues associated with synthetic nitrogen fertilizers,” emphasized the research team, led by senior author Jean-Michel Ané of the University of Wisconsin–Madison.
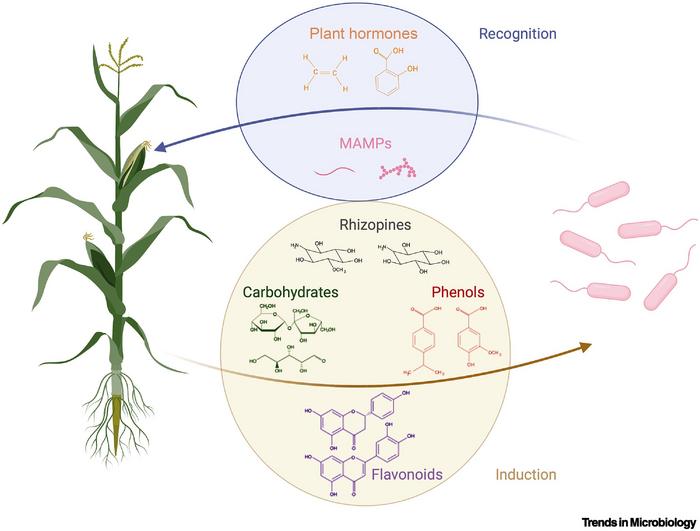
Diazotrophs, a class encompassing soil bacteria and archaea, possess the innate ability to "fix" atmospheric nitrogen into ammonium—a nitrogen source available to plants. Some of these microorganisms have formed mutually beneficial relationships with plants, with the latter furnishing a source of carbon and a sheltered, low-oxygen refuge in exchange for a precious nitrogen supply. For example, leguminous plants host nitrogen-fixing microbes within small nodules on their root systems.
However, these intricate partnerships manifest solely within a limited array of plant species, rendering them ill-suited for mainstream agricultural applications. However, these sorts of relationships take eons to evolve naturally.
An array of methods have been proposed to enhance nitrogen fixation in non-legume crops, including genetic modification to endow plants with the capacity to produce nitrogenase—the enzyme responsible for converting atmospheric nitrogen into ammonium—or engineering non-legume plants to foster root nodules.
The alternative approach, central to this review, hinges on the genetic alteration of both plants and nitrogen-fixing microbes to cultivate symbiotic bonds. Plants would be engineered to become more hospitable hosts, while microbes would be primed to release fixed nitrogen more readily in response to molecules secreted by their engineered plant hosts.
The research team elucidated, “Since free-living or associative diazotrophs do not altruistically share their fixed nitrogen with plants, they need to be manipulated to release the fixed nitrogen so the plants can access it.”
This approach would require a bidirectional signaling system between plants and microbes, a phenomenon that already occurs naturally. Microbes deploy chemoreceptors to detect metabolites secreted by plants into the soil, while plants sense microbe-associated molecular patterns and microbe-secreted plant hormones. Genetic engineering could fine-tune these signaling pathways to make communication more specific between pairs of engineered plants and microbes.
Efficiency optimization also assumes a pivotal role in this endeavor. Nitrogen fixation is an energetically demanding process, so the ability of microbes to regulate nitrogen fixation and only produce ammonium when necessary is very valuable. “Relying on signaling from plant-dependent small molecules would ensure that nitrogen is only fixed when the engineered strain is proximal to the desired crop species,” the authors write. “In these systems, cells perform energy-intensive fixation only when most beneficial to the crop.”
Beyond nitrogen fixation, numerous nitrogen-fixing microbes hold the potential to provide additional benefits to plants, including growth stimulation and enhanced stress tolerance. The authors underscore the necessity of future research endeavors to explore the prospect of ‘stacking’ these varied advantages. However, due to the energy-intensive nature of these processes, the authors suggest the creation of microbial communities comprising diverse species, each contributing distinct benefits to "distribute the production load among several strains."
Nevertheless, the authors are aware of the complex nature of genetic modification in the public eye. The widespread deployment of genetically modified organisms in agriculture hinges on public acceptance. “There needs to be transparent communication between scientists, breeders, growers, and consumers about the risks and benefits of these emerging technologies,” the authors write.
Biocontainment emerges as yet another pertinent issue. Given the propensity of microbes to interchange genetic material within and across species, stringent measures are imperative to prevent the dissemination of transgenic material into native microbes in nearby ecosystems. Various biocontainment strategies have been developed, such as engineering microbes to rely on molecules not naturally available or incorporating "kill switches." The authors posit that the efficacy of these control mechanisms might be improved through layering, as each mechanism has its limitations. Rigorous testing of these engineered plant-microbe mutualisms under the variable field conditions in which crops are grown is strongly recommended.
The authors argue, "The practical use of plant-microbe interactions and their transition from laboratory settings to real-world agricultural contexts remains challenging due to the extensive variability in biotic and abiotic environmental factors and their repercussions on plants, microbes, and their interactions. Trials conducted in highly controlled environments, such as greenhouses, often exhibit poor translation to field conditions. We propose that engineered strains should be subjected to more readily available and highly replicated field trials."


















