Ai Digital Biology
DNA Folding Technique Opens Array of Possibilities for Molecular Biology & Nanotech
Jun 19, 2023
Credit: Colourbox
In the teeming microcosm of a human cell, about 2 meters of DNA—packed with our unique genetic code—is tightly bundled into chromosomes. In a stunning display of nature's design, if one were to unravel the DNA within a single person, it would cover a distance equivalent to 60 round trips to the sun.
Minke A.D. Nijenhuis, a co-corresponding author of the study, paints an apt metaphor: “Imagine DNA as a piece of paper upon which all our genetic information is written. The paper is folded into a very tight structure to fit all that information into a small cell nucleus. To read the information, parts of the paper have to be unfolded and then refolded. This spatial organization of our genetic code is a central mechanism of life. We, therefore, wanted to create a methodology that allows researchers to engineer and study the compaction of double-stranded DNA.”
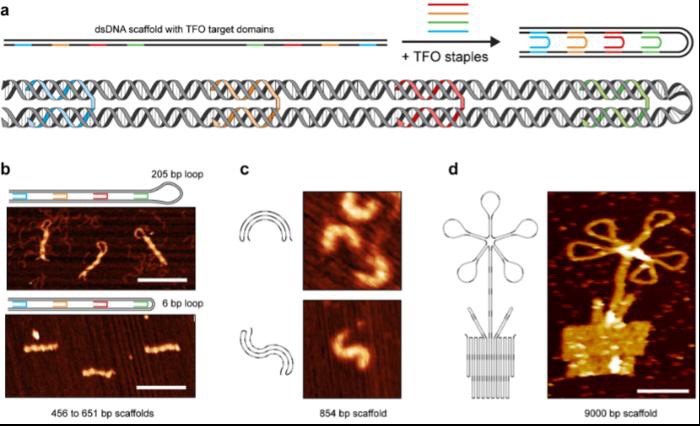
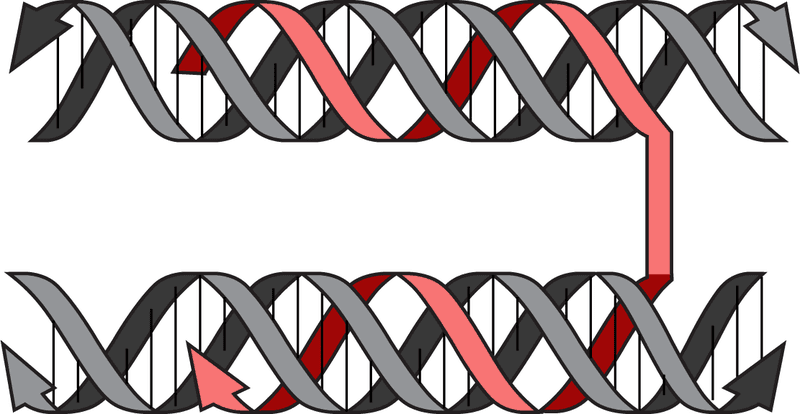
In a groundbreaking study published in Advanced Materials, researchers led by Professor Kurt V. Gothelf unveiled a new technique for manipulating double-stranded DNA based on Hoogsteen interactions. These interactions allow a third DNA strand to intertwine with the typical double helix, forming a triple helix.
Researchers discovered that this triple helix provides protection and compactness to the DNA. Through a method named 'triplex origami,' researchers demonstrated that triplex-forming strands could sharply bend double-stranded DNA into various shapes and structures—ranging from hollow two-dimensional figures to dense three-dimensional constructs. The triplex origami technique also presents significant potential in understanding the natural process of genetic DNA compaction.
“Long dsDNA templates were folded into designed shapes via triplex-mediated self-assembly. Triplex-forming oligonucleotides (TFOs) bind purines in dsDNA via normal or reverse Hoogsteen interactions,” the authors penned. “In the triplex origami methodology, these non-canonical interactions are programmed to compact dsDNA (linear or plasmid) into well-defined objects, which demonstrate a variety of structural features: hollow and raster-filled, single- and multi-layered, with custom curvatures and geometries, and featuring lattice-free, square-, or honeycomb-pleated internal arrangements. Surprisingly, the length of integrated and free-standing dsDNA loops can be modulated with near-perfect efficiency, from hundreds down to only six bp (2 nm).”
This newfound control over DNA's structure could have far-reaching implications in gene therapy, where diseased cells are repaired by inserting functional genes through deliverable double-stranded DNA. The Hoogsteen-mediated triplex formation shields the DNA from enzymatic degradation, providing enhanced protection and compactness with the triplex origami method.
Nanoscale materials engineering has also benefitted from this revelation in DNA sequence and structure, offering advancements in therapeutics, diagnostics, and other related areas. According to Professor Gothelf, “For the past four decades, DNA nanotechnology has almost exclusively relied on Watson-Crick base interactions to pair up single DNA strands and organize them into custom nanostructures. We now know that Hoogsteen interactions have the same potential to organize double-stranded DNA, which presents a significant conceptual expansion for the field.”
Interestingly, the triplex origami structures formed through Hoogsteen-mediated folding were compatible with conventional Watson-Crick-based methods. In fact, due to the comparative rigidity of double-stranded DNA, these structures require fewer starting materials—leading to larger structures at a significantly reduced cost.
However, the technique isn't without its limitations. The formation of a triplex requires long stretches of purine bases within the double-stranded DNA. Therefore, researchers currently use artificial DNA sequences but are looking to overcome this hurdle in the future. As we venture into this brave new world of DNA manipulation, the mystery of our genetic code continues to unravel, promising unprecedented opportunities in gene therapy and beyond.


















