Carbon-Capturing Cultures: Marine Microbes in the Fight Against Greenhouse Gases
An innovative enzyme-electrode duo efficiently transforms CO2 into formate, offering a new method for carbon capture and green energy storage
Oct 3, 2023
Francesceo Ungaro (Canva)
In collaboration with experts from Radboud University and the University of Geneva, scientists at the Max Planck Institute for Marine Microbiology are forging a pioneering path in the quest to combat climate change. In a paper published in Angewandte Chemie, the team details the process of seeking out microorganisms with the ability to efficiently capture the notorious greenhouse gas, carbon dioxide (CO2).
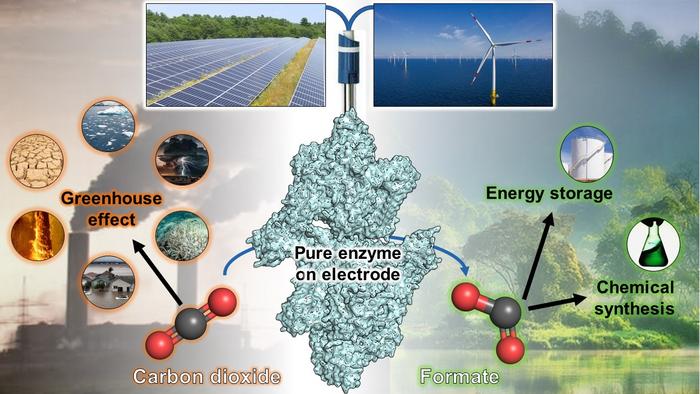
Leader of this research and head of the Max Planck Research Group Microbial Metabolism Tristan Wagner underscores the pivotal role of enzymes employed by these microorganisms, describing them as a "fantastic playground for scientists" due to their capacity to facilitate highly specific reactions at astonishing rates. These enzymes are catalysts for a unique CO2-capturing process that transforms the gas into formate, a stable compound with versatile applications in energy storage and the synthesis of industrial and pharmaceutical molecules.
One of the prime examples of such microorganisms is Methermicoccus shengliensis, a methane-producing microbe isolated from an oilfield and cultivated by Julia Kurth and Cornelia Welte at Radboud University. At the Max Planck Institute for Marine Microbiology, Olivier Lemaire, Mélissa Belhamri, and Tristan Wagner have dissected this microbe's secrets, specifically its CO2-capturing enzyme and its efficiency in transforming CO2.
Isolating the microbial enzyme responsible for the transformation proved a formidable challenge. “Since we knew that such enzymes are sensitive to oxygen, we had to work inside an anaerobic tent devoid of ambient air to separate it from the other proteins – quite complicated, but we succeeded”, says Olivier Lemaire. Once isolated, the researchers meticulously characterized the enzyme, revealing its remarkable efficiency in generating formate from CO2. “Similar enzymes belonging to the family of formate dehydrogenases are well known to operate in both directions, but we showed that the enzyme from Methermicoccus shengliensis is nearly unidirectional and could not efficiently convert the formate back into CO2”, reports Mélissa Belhamri. “We were quite thrilled by this phenomenon, occurring only in the absence of oxygen”, she adds.
“Since the formate generated from CO2-fixation cannot be transformed back and therefore accumulates, such a system would be a highly interesting candidate for CO2-capture, especially if we could branch it on an electrode”, Tristan Wagner points out. By naturally or chemically attaching the enzyme to an electrode, the energy required for CO2 capture is directly supplied by the electrode, eliminating electric current losses and the need for costly or toxic chemical compounds. These enzyme-bound electrodes demonstrate remarkable efficiency in gas conversion procedures, and as such, the purified enzyme was dispatched to the University of Geneva to establish an electrode-based CO2-capture system.
Selmihan Sahin and Ross Milton, specialists in electrochemistry at the University of Geneva, sought to replace polluting and rare metals commonly used in electrode-based formate generation from CO2 with the enzyme extracted by Tristan Wagner's research group. The process of enzyme binding to an electrode, while not always straightforward, was facilitated by the unique characteristics of the enzyme. Sahin and Milton successfully anchored the enzyme to a graphite electrode, achieving gas conversion rates comparable to traditional formate dehydrogenases. Again, the system exhibited poor performance in the reverse reaction, consistent with earlier observations in the reaction tube. As a result, the modified electrode consistently transformed greenhouse gas into formate without generating detectable side-products or incurring electric current loss.
This collaborative research effort provides the scientific community with a powerful molecular tool—an enzyme capable of converting CO2 into formate with exceptional efficiency when coupled with electricity. Renewable green energy sources such as wind and solar power could supply the necessary electricity to this electrode-based system, ultimately converting CO2 into a directly usable molecule or a means of energy storage. While the potential for large-scale CO2 transformation is alluring, it comes with the challenge of scaling up enzyme production systems, a substantial undertaking. Thus, a comprehensive understanding of the enzyme's mechanism remains crucial before its practical application can be realized. The research team is poised to delve deeper into the molecular intricacies of this transformative process, paving the way for a potential game-changer in the battle against atmospheric CO2.


















