Building Microbiomes From Scratch
The development of synthetic microbiomes represents a significant leap forward in biotechnology, with potential applications ranging from infection prevention to cancer treatment
[DALL-E]
In 2022, Alice Cheng, Michael Fischbach, and their colleagues published a paper documenting the creation of a synthetic human microbiome for the first time. This microbiome consisted of 119 species sourced from bacterial species naturally found in humans. They carefully cultured these species, transferred all species together into germ-free mice, and found that they could stably colonize mice and fend off pathogenic Escherichia coli infection.
Synthetic microbial communities are assemblies of microbes isolated from human samples. Historically, these communities have been used to interrogate experimental questions like how do the microbiome and host interact, what species contribute to resilience to infections, and how can the microbiome be modulated to treat other diseases. But these and other similar microbial systems are beginning to make their way into therapeutic spaces.
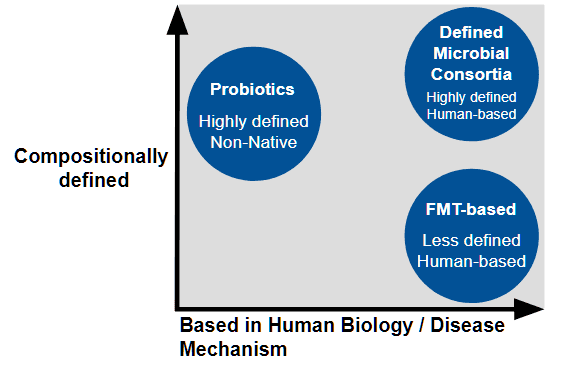
What’s In a Name? Synthetic Microbiomes, FMTs, and Probiotics
“When Alice and I envisioned this project, the idea that you could build a community that large and that it would behave reproducibly and stably was just outlandish,” says Fischbach, Professor of Bioengineering at Stanford University.
“I really wanted to get a more standardized fecal transplant. That was my driving motivation,” says Cheng, Assistant Professor at the University of Chicago Medicine..
Although microbial communities in fecal transplants represent the totality of functions carried out by the microbiome, they aren’t compositionally defined. “As a therapeutic or even an experimental substrate, it's not great because you want as much control over what you're giving or experimenting on as possible,” says Cheng.
In efforts to create a more standardized microbiome treatment, the clinical stage company MaaT Pharma found that pooling the microbiome from several healthy donors results in a more homogeneous and diverse microbiome. “Pooling holds the potential to standardize the product composition, with a significant reduction of variance within manufactured pooled products,” says Hervé Affagard, CEO of MaaT Pharma.
Other microbiota-based therapies aimed at modulating the microbiome, including probiotics or existing defined microbial consortia containing very few species, are compositionally defined but can lack the number of species needed to replicate human biology. It’s possible that approaches that focus on a single strain or small consortia might miss out on the full complexity of the microbiome, says Affagard.
Currently the only FDA approved microbiome therapeutic available are based on donor fecal material are Vowst, from Seres Therapeutics, and Rebyota, from Ferring Pharmaceuticals, to prevent recurrent Clostridioides difficile infection.
While Cheng and Fischbach’s synthetic community consists of a large number of organisms, that doesn’t mean it’s unwieldy. “That's an interesting lesson for the design and engineering of complex systems. It doesn't always make the system less manageable to make it more complex,” says Fischbach.
Overcoming Logistical Challenges of Growth
However, there are still challenges in developing a diverse and defined microbial community. For Cheng and Fischbach, this meant growing 119 microorganisms from scratch. “A lot of logistic work has to be done to optimize the growth of very complex consortia or artificial microbiomes for patients,” says Cheng.
On the other hand, this complexity also means scientists have more control and can optimize the microbiome for certain purposes. “We can decide who's in and who's out,” says Fischbach. Since strains in synthetic communities are selected one by one, it’s possible to engineer community members for specific therapeutic goals before introducing them into the synthetic community. “You could drop constructs in to display an immune-activating molecule on their surface or secrete metabolite that's biologically important,” says Cheng.
Because the organisms naturally grow together, it’s possible that they can be grown as a consortium to simplify manufacturing. This is something that the live biotherapeutics company Kanvas Bio is working on. As part of their workflows, they manufacture microbial consortia starting from purified cell lines and co-culture communities of these strains at an industrial scale.
Therapeutic Applications in Clinical Trials
While these types of large synthetic microbiomes are quite new, much of the work in the industry has focused on smaller consortia for therapeutics, says Cheng.
Vedanta Biosciences has had success in a Phase 2 clinical trial using a live bacterial consortium of 8 bacterial strains of commensal Clostridia to treat adults with repeated Clostridioides difficile infections. They’re also piloting other bacterial consortia to prevent multidrug-resistant infections and treat ulcerative colitis.
Aside from their pooled microbiome therapies in clinical trials, MaaT Pharma is investigating co-cultured, indication-specific treatments. Their co-culture microbiome ecosystem therapies (METs) are based on AI-driven analysis of data from healthy individuals and patients, says Affagard. This allows them to construct METs on a large scale that mimics the richness and diversity of native-based microbiomes. One of these treatments, MaaT03X, consists of hundreds of co-cultured species.
Last year, the now-defunct Federation Bio tested a 148-member synthetic microbiome in a phase 1 clinical trial to treat enteric hyperoxaluria, which is characterized by increased absorption of oxalate in the gut. Kanvas Bio, which acquired the consortium from Federation Bio, is now repurposing it to treat IBD. This application is undergoing phase 1 clinical trials.
Modulating the Microbiome to Treat Beyond the Gut
Many therapeutics being developed use microbial consortia to treat problems that stem from the microbiome. However, there are many other therapeutic areas that aren’t necessarily problems arising from the microbiome but can be treated with an engineered microbial community, says Fischbach. One of these areas is the immune system, where the microbiome can “very precisely and potently manipulate the immune system,” says Fischbach.
This is something that MaaT Pharma is exploring. “Since our inception ten years ago, we have been committed to leveraging microbiome-based therapies to restore immune homeostasis in cancer patients, potentially reshaping cancer treatment paradigms and positioning microbiome therapies as the fifth pillar of cancer treatment,” says Affagard. “As of today, including all clinical trials and early access program, we have safely treated more than 300 patients.”
Companies like SNIPR Biome and Eligo Bioscience are taking a different approach to microbiome modulation by editing the microbiome using CRISPR to selectively eliminate bacteria from the microbiome with applications in oncology, immunoinflammation, infectious disease, and more.
“We are witnessing a transformative and maturing phase within our field as it has been the case at the beginning of the DNA and human genome sequencing,” says Affagard. “This dynamic reflects the emergence of a new field within the biotech industry.”
“It feels like something is about to open up in terms of our ability to understand and engineer the microbiome,” says Fischbach.


















