Engineered Human Therapies
Brain Tumor Organoids Mirror Glioblastoma Responses to CAR T
Penn researchers harness tumor organoids to personalize CAR T cell therapy for glioblastoma patients
Dec 9, 2024
[Grok]
Researchers at the University of Pennsylvania's Perelman School of Medicine have developed lab-grown tumor organoids to model patient-specific responses to CAR T cell therapy in real time. Published in Cell Stem Cell, the study revealed that the organoids—derived directly from glioblastoma (GBM) tumors—accurately predicted how individual patients’ brain tumors would respond to treatment.
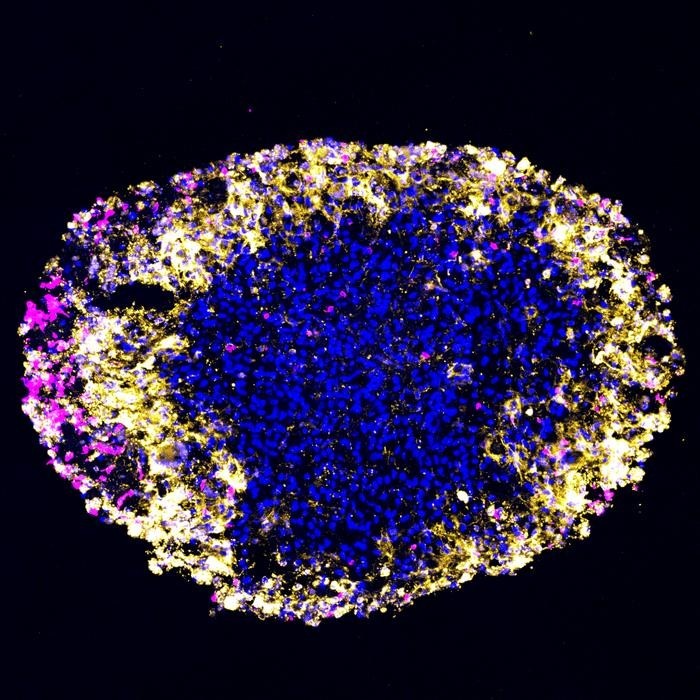
The organoids served as a reliable proxy: when the lab-grown tumors shrank under therapy, so did the corresponding tumors in patients’ brains. “We face significant challenges in monitoring GBM treatment because regular biopsies are impossible, and MRI imaging often blurs the line between tumor growth and inflammation caused by treatment,” explained Hongjun Song, PhD, Perelman Professor of Neuroscience and co-senior author. “These organoids replicate what’s happening in the patient’s brain with remarkable accuracy. We hope they’ll enable rapid identification of effective therapies tailored to each patient’s tumor.”
The Challenge of Treating Glioblastoma
GBM is both the most common and the most aggressive type of brain cancer in adults. The prognosis remains grim, with life expectancy averaging 12–18 months after diagnosis. Standard treatments like surgery, radiation, and chemotherapy have limited efficacy, and decades of research have yet to yield a cure.
CAR T cell therapy, a promising immunotherapy, involves engineering a patient’s T cells to recognize and destroy specific cancer cells. While the therapy has revolutionized treatment for certain blood cancers, researchers have struggled to achieve similar success with solid tumors like GBM. A recent innovation—dual-target CAR T cell therapy, which attacks two tumor-associated proteins—has shown potential for shrinking GBM tumors.
Organoids: A Game-Changer for Modeling Brain Tumors
“GBM tumors are incredibly complex, consisting of multiple cancer cell types, immune cells, blood vessels, and other tissues,” said Guo-li Ming, MD, PhD, co-senior author and Perelman Professor of Neuroscience. “By growing organoids from a patient’s actual tumor, we replicate not only the tumor’s cellular makeup but also its surrounding microenvironment—a critical limitation of traditional models.”
For this study, researchers created organoids using tumor samples from six patients with recurrent GBM participating in a Phase I trial for dual-target CAR T cell therapy. Unlike conventional methods that require months to culture sufficient cancer cells, organoids can be generated in just 2–3 weeks—allowing researchers to test therapies while patients recover from surgery and prepare for treatment.
Mirroring Treatment Responses
In a breakthrough experiment, organoids and patients received CAR T cell therapy simultaneously, 2–4 weeks after surgery. The organoids’ responses mirrored those of the patients: when organoids showed tumor cell destruction, patients exhibited reduced tumor sizes on MRI scans and increased CAR-positive T cells in their cerebrospinal fluid, indicating successful targeting of the tumor.
One critical concern with CAR T therapy for GBM is neurotoxicity, which can disrupt or kill brain cells. The researchers observed parallel levels of immune cytokines—biomarkers of toxicity—in both the organoids and patients’ cerebrospinal fluid. Importantly, these levels decreased a week after treatment, underscoring the organoids’ potential to model neurotoxicity risks and guide dosage decisions.
Toward Personalized GBM Treatment
“This research demonstrates that our GBM organoids are an accurate and powerful tool for understanding how CAR T therapy impacts brain tumors,” said Donald M. O’Rourke, MD, co-senior author and director of the Glioblastoma Translational Center of Excellence. “Our goal is to bring these organoids into clinical use, enabling personalized treatment strategies while advancing our understanding of how to combat this complex and deadly cancer.”
By leveraging organoids, researchers hope to overcome one of the greatest challenges in GBM treatment: its immense complexity. These lab-grown models may hold the key to outsmarting a disease that has long defied medical science.


















