A CAR-T Cell Side Project Takes Center Stage Offering Hope Beyond Cancer
How a graduate student's curiosity led to a two-year breakthrough journey in CAR-T-cell therapy at MUSC Hollings Cancer Center
Sep 13, 2023
Marcin Klapczynski (Canva)
Two years ago, Dr. O’Neil embarked on a mission to improve CAR-T-cell therapy at the MUSC Hollings Cancer Center. Little did he know that a side project initiated by Megan Tennant, a graduate student in his lab, would lead to groundbreaking results.
CAR-T-cell therapy, currently used to combat certain recurrent blood cancers unresponsive to chemotherapy, is known for its high cost and extensive procedures. Patients undergoing this therapy have had remarkable recoveries but often endure severe and frightening side effects, primarily from lymphodepletion chemotherapy administered prior to CAR-T-cell reinfusion.
However, Dr. O’Neil and his team, including Dr. Christina New and Dr. Leonardo Ferreira, challenge these side effects. Their research, published in Molecular Therapy, demonstrates that encoding CAR-T-cells with instructions to activate a hyperactive form of protein STAT5 can promote cell engraftment without requiring lymphodepletion. Importantly, this engraftment process occurs autonomously within the cells, offering a more efficient and less invasive alternative.
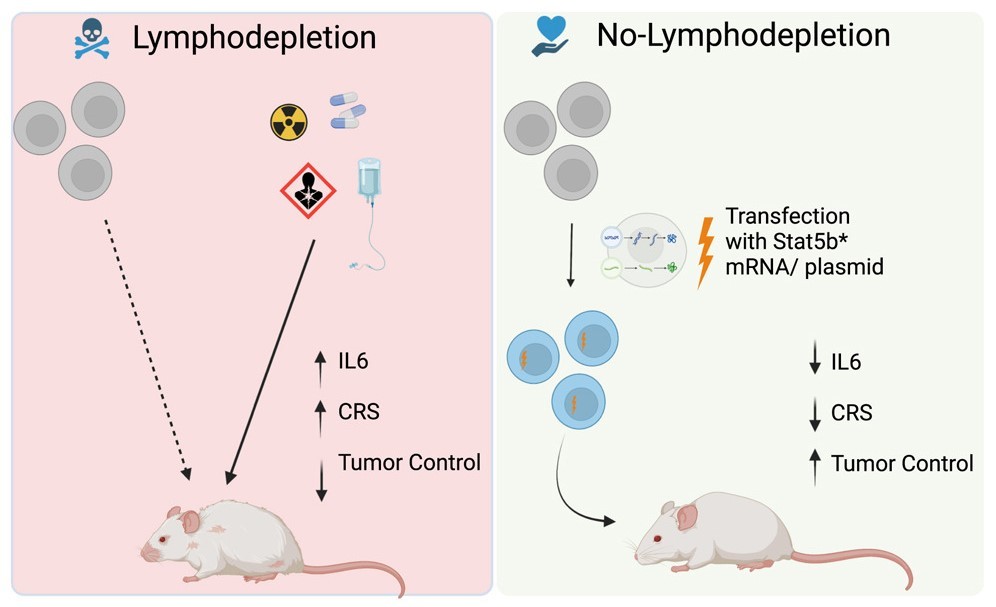
“We present a lot of evidence in the paper to support that notion that it's a completely cell autonomous process and that it's fundamentally driven by activation of STAT5,” O’Neil explained. “And so by transiently activating STAT5 during that phase of adoptive transfer, the initial engraftment phase, you're tricking the cells into essentially thinking they're going into a lymphodepleted environment."
Tennant, reflecting on the unexpected success, said, “I don't think that either of us expected that first initial experiment to work. But when we saw how well it worked and really started to conceptualize where this could go and how important this could be, it was exciting.”
By eliminating the need for lymphodepletion, the researchers aim to broaden the applicability of CAR-T-cell therapy to a wider range of diseases, including chronic conditions like lupus. O’Neil envisions the possibility of administering multiple doses to patients instead of the current "one and done" approach, which could significantly impact treatment schedules.
The team utilized messenger RNA transfection to implant the instructions for activating STAT5 within the CAR-T-cells. In preclinical models, this method not only reduced cytokine release syndrome, one of the most severe side effects, but also effectively controlled cancer and generated memory cells specifically trained to target it.
Dr. O’Neil is now collaborating with the MUSC lupus erythematosus research group to potentially develop clinical trials. Additionally, discussions with biotechnology companies specializing in CAR-T-cell therapy are underway to initiate new clinical trials.
He commended Megan Tennant for her dedication to the project, stating, “This has been Megan's labor of love...this entire project came from conception to fruition in two years, which is pretty amazing and definitely shows what a talented and hard-working scientist Megan is.”