Flagship Pioneering announced the launch of its newest biotech firm, Sigilon Therapeutics on Wednesday, June 21st, with a $23.5 million round of financing and is the latest company in a line of Cambridge/Boston biotechs to come out of VentureLabs, Flagship’s in-house innovation foundry. Sigilon Therapeutics is developing treatments for chronic diseases using new biomaterials, developed at the Massachusetts Institute of Technology (MIT), that can shield implanted cells from immune attack. The company’s mission is to discover and develop category-defining biocompatible encapsulated cell therapies.
The company’s platform implements cell engineering and biocompatible AfibromerTM technology,a new class of implantable biomaterials developed with the goal of delivering cell therapies without triggering fibrosis, a pervasive problem with encapsulated cell therapies. Fibrosis, the scarring of tissue, is the immune system’s response to what the system perceives as a foreign material.
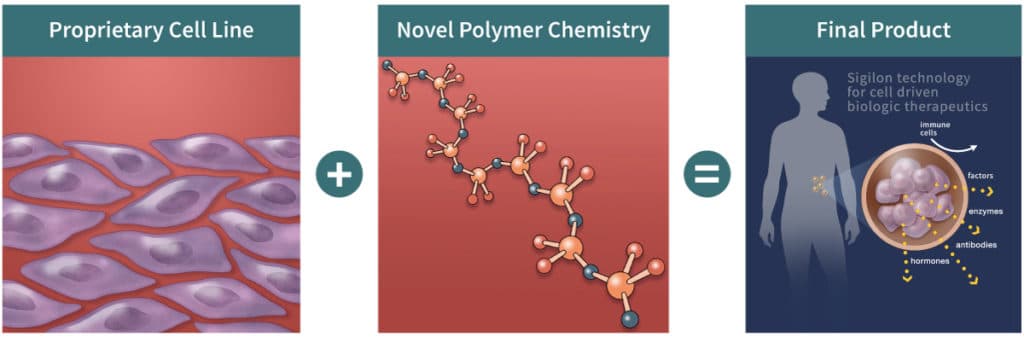
Sigilon Therapeutics is pioneering technologies that will harness the full power of cell therapeutics. Source: http://sigilon.com/technology-platform/Afibromer capsules, despite being in the preclinical stage, have demonstrated revolutionary cell survival and function for extended periods. Sigilon’s proprietary cells have been designed to secrete therapeutic proteins in combination with the Afibromer™ technology. These implanted cells are hidden from the immune system allowing levels of the missing protein in the body to be restored for long periods of time following a single implantation.

Polystyrene beads coated with Afibromer™ technology vs uncoated beads. When these beads are implanted into an animal, there is rapid fibrosis of the uncoated beads, while the Afibromer™ coated beads remain pristine. Source: http://sigilon.com/technology-platform/

The company will develop products that emerge from its discovery platform to treat serious hematologic, enzyme deficiency and endocrine disorders. Source: http://sigilon.com/technology-platform/These capsules, with their ability to deliver proteins over an extended period of time, eliminate the need for traditional therapies, such as series of injections or pill regimens, improving patients’ quality of life. One of the many pluses of this therapy as compared to experimental gene therapy or gene editing is that the capsules can be removed if there are concerns about their safety or effectiveness. Sigilon plans to initially focus on therapies to treat blood, enzyme deficiency and endocrine disorders.In the press release, Sigilon Founding Chairman of the Board and Flagship Pioneering Managing Partner, Doug Cole, stated: “Harnessing the power of cells to treat diseases has been a holy grail for medicine since the advent of biotechnology. It opens the possibility of treating patients with serious illnesses without the risks of immunosuppression or genetic manipulation.” “Following two years of forming the innovation and IP foundations within Flagship VentureLabs, Sigilon Therapeutics is poised to leverage its unique approach to engineering controllable and dose-adjustable cell systems to provide a natural, effective form of delivery that vastly expands therapeutic options for patients and physicians.”Professor Robert Langer, one of the co-founders and a director of Sigilon Therapeutics, commented on Sigilon’s product line: “Restoring critical proteins in the body in an effective and controllable way has been an elusive goal for many years. The discovery of permeable biomaterials that avoid fibrosis opens a broad range of possibilities. Sigilon Therapeutics’ technology allows implanted cells to deliver proteins in a controlled manner over extended periods and can be envisioned as a transplanted tissue that avoids effects of rejection and isolation by the immune system.”Paul K. Wotton, Ph.D., CEO and member of the board of directors, said the following about the benefits of the Sigilon approach: “Imagine the potential of a ‘living therapeutic’ that could be implanted in the body and manufacture and release therapeutic proteins at steady levels for long periods of time, avoiding the critical limitations of intermittent infusion required with current therapies.”Flagship PioneeringFlagship Pioneering conceives, creates, resources and develops first-in-category life sciences companies. The firm has founded and funded some of the most innovative companies in biotechnology over the last two decades.Since its inception in 2000, Flagship has raised $1.75 billion and has launched approximately 100 scientific ventures with a resulting $20 billion plus in aggregate value, over 500 patents and more than 45 clinical trials for newly developed therapeutic agents.